

Yankin shrinkage na kowane tsawon rukunin a farfajiya na ruwa ana kiransa tashin hankali mai ƙarfi, kuma naúrar ita ce N.TH M-1.

Dukiyar rage tashin hankali na saman da sauran ƙarfi ana kiransa ayyukan ƙasa, kuma wani abu tare da wannan kayan ana kiransa abu mai aiki.
Abubuwan da ke aiki da ƙasa wanda zai iya ɗaure kwayoyin halitta a cikin mafita da kuma tsari na mayu da sauran ƙungiyoyi, kuma suna da tasirin sama, kuma suna da rawar jiki, wanka, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wankewa, wanka.
Surfactant shine mahadi na kwayoyin halitta tare da tsari na musamman da dukiya, wanda zai iya canza mahimmancin hade tsakanin matakai biyu (gaba ɗaya), tare da rigar ruwa, wankewa da sauran kaddarorin.
Dangane da tsari, Sepactants suna da fasalin gama gari a cikin cewa sun ƙunshi ƙungiyoyi biyu na dabi'a a cikin kwayoyin halitta. A ƙarshen ɗaya shine dogon sarkar rukunin ba polar, wanda ke narkewa a cikin mai da kuma wanda ba a san shi da ƙungiyar hydrophobic ko rukunin hayaƙi ba. Irin wannan rukunin masu sauraro ne gabaɗaya ne na hydrocarbons, wani lokacin kuma don fruorine na Orgichle, rukunin Orgichilic, ƙungiyar masu lalata. Rukunin Hydrophilic dole ne ya isa hydrophilic ya isasshe hydrophilic don tabbatar da cewa duka surfactants suna da narkewa a cikin ruwa kuma yana da gaiyan da ya dace. Tunda Surfactants dauke da kungiyoyin hydrophobic da hydrophobic, za su iya zama mai narkewa a akalla ɗaya daga cikin matakai na ruwa. Wannan kayan aikin hydrophilic da lipphilic na Surfactant ana kiran shi ne AMIPHIIty.


Surfactant wani nau'in kwayoyin ne ampilic tare da ƙungiyoyi biyu masu hydrophilihili. Hukumar Hydrophobic na Surfactants duka suna da dogon hydrocarbons, irin su madaidaiciya-sarkar alkyl c8 ~ c20 Tom lamba shine 8 ~ 16) da makamantansu. Bambanci wanda yake ƙarami tsakanin ƙungiyoyin Hydrophobic shine mafi yawan canje-canje na tsarin hydrocarbon sarƙoƙi. Kuma nau'ikan kungiyoyin Hydrophilic sun fi yawa, don haka kaddarorin surfactants galibi suna da alaƙa da girman Hydrophilic ban da girman da siffar ƙungiyoyi na hydrophobic. Hanyoyin canje-canje na kungiyoyin Hydrophilic sun fi na kungiyoyin hydrophobic fiye da yadda aka tsara su na Surfactants gabaɗaya bisa tsari na kungiyoyin Hydrophilic. Wannan rarrabuwa ya dogara da ko kungiyar hydrophilic shine ionic ko a'a, kuma an kasu kashi anicic, cātroc, da ba a raba shi da sauran nau'ikan surfactants na musamman.

① AdsorPtion of Surfactants a Interfac
Kwayoyin halitta sune kwayoyin halittun masu amsawa da suke da su biyu da kuma kungiyoyin hydrophili. Lokacin da Surfactant ke narkar da ruwa, ƙungiyar hydrophilic tana jan hankalin ruwa da ruwa da kuma barin ɗakunan ruwa, wanda ke rage tashin hankali ta hanyar matakai biyu, wanda ke rage tashin hankali tsakanin matakai biyu. Mafi kyawun kwayoyin halitta (ko ions) ana tallatawa a cikin dubawa, mafi girman raguwa a cikin tashin hankali na Interfacial.
② wasu kaddarorin membrane
Matsakaitaccen matsin lamba na Membrane: adfactant adsorption a cikin binciken da ke dubawa don samar da matsi a kan takardar mai ta, wanda ake kira matsin lamba.
Keɓaɓɓen Keɓaɓɓen: Kamar matsin lamba, danko farfajiya shine dukiyar da ba ta dace ba ta hanyar Membrane. An dakatar da kyawawan ƙarfe na ƙarfe na Platinum na Platinum, don tauraronsa yana lalata ruwan sha, jujjuya saƙo a hankali, a gwargwadon zobe za'a iya auna shi. Hanyar ita ce: ta farko, ana gudanar da gwajin a kan tsarkakakken ruwa don auna ƙimar ƙwayar ƙwayar ƙwayar ƙasa, sannan kuma danko na ƙasa da keɓaɓɓe tsakanin su biyu.
Dangane da farfajiya yana da alaƙa da ƙarfi na Membrane, kuma tun lokacin da membraneption na adsorption yana da matsin lamba da danko, dole ne ya kasance yana da elasticity. Mafi girman matsin lamba kuma mafi girma danko na adsorbed membrane, mafi girma m modulus. Modulus na roba na metorPar na saman adsempane yana da mahimmanci a cikin aiwatar da haɓakar haɓakawa.
③ samuwar micelles
Dripou Sallutions na Sucfacts suna yin biyayya da dokokin da ya biyo baya. Yawan Surfactant Adsorbed a saman mafita na ƙara tare da taro na mafita, kuma lokacin da aka maida hankali ko ya wuce wani bayani a cikin hanyar Haphazard. Dukansu suna da ka'idar duka sun nuna cewa suna haifar da ƙungiyoyi cikin mafita, kuma ana kiran waɗannan ƙungiyoyi Mogeles.
M ƙwarewar micelle mai mahimmanci (CMC): Mafi ƙarancin taro a wanne surfactants ya samar da miceles a cikin bayani ana kiranta mahimmancin micelle maida hankali.
④ cmc dabi'u na Surfacts na kowa.

HLB shine raguwa na daidaitaccen lamuni, wanda ke nuna daidaituwar hydrophilic da lippophilic rukuni na surfactant, watau, darajar HLB na surfactant. Babban darajar HLB yana nuna kwayoyin tare da ƙwayoyin cuta da rauni mai rauni; Conversely, mai ƙarfi lipophilicity da rauni hydrofity.
① tanadi na darajar HLB
Darajar HLB ce ƙimar dangi, don haka lokacin da aka haɓaka darajar HLB, a matsayin daidaitaccen darajar Surfactant, wanda ya fi ƙarfin hydrochilic, shine 40 saboda magana da shi, emulsifiers tare da Haɗin HLB kasa da 10 sune lipophilic, yayin da waɗanda suka fi 10 girma hydrophilic. Don haka, juyawa daga Lipophilic zuwa Hydrophilic shine kusan 10.
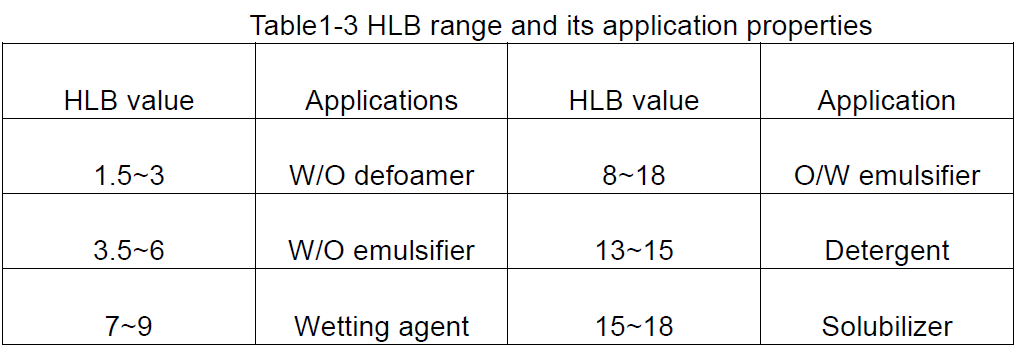
Dangane da ƙimar HLB na Surfactants, ana iya samun cikakken ra'ayin gaba ɗaya game da amfani, kamar yadda aka nuna a Tebur 1-3.

Two mutually insoluble liquids, one dispersed in the other as particles (droplets or liquid crystals) form a system called an emulsion. Wannan tsarin shine m ismindynamical wanda ba zai iya m saboda karuwa a cikin yankin yanki na ruwa na ciki lokacin da emulsion aka kafa. Domin sanya emulsion bargion, ya zama dole don ƙara kayan haɗin uku - emulsifier don rage ƙarfin shiga cikin tsarin. Emulsifier nasa ne na Surfactant, babban aikinsa shine wasa da emulsion. Lokaci na emulsion da ke kasancewa kamar yadda ake kiran droplets da aka tarwatsa (ko kuma wani lokaci na ciki, dakatar da lokaci wanda aka haɗa tare da matsakaiciyar matsakaici (ko lokaci na waje, ci gaba lokaci.
①ulsifiers da emulsions
Emulsions gama gari, lokaci guda shine ruwa ko mafi tsummoki, da man shafawa da ruwa don samar da ruwa mai-ruwa, wanda aka bayyana a matsayin W / o (ruwa / man). Tsabtattun ruwa-in-man-mai-mai-mai-mai-mai-mai-mai-mai da-ruwa-in-mai-mai-ruwa mai-mai-ruwa ma za'a iya kafa shi.
Emulsifiers ana amfani da su don daidaita emulsions ta hanyar rage tashin hankali na Interfacial da kuma samar da membrane na membulane guda ɗaya.
A cikin emulsification na buƙatun emulsifier:
A: emulsifier dole ne ya iya yin adorb ko kuma yana wadatar da keɓance tsakanin matakai biyu, don haka ya rage tashin hankali na InterFacaial;
B: Mai emulsifier dole ya ba da barbashi zuwa cajin, saboda haka karfin lantarki tsakanin barbashi, ko siffofin karuwa, mai kariya ta kariya a kusa da barbashi.
Saboda haka, kayan da ake amfani da shi azaman emulsifier dole ne ya sami ampulhilic ƙungiyoyi domin emulsify, da kuma surfactants na iya biyan wannan buƙata.
Hanyoyin emulsions da abubuwan da suka shafi kwanciyar hankali na emulsions
Akwai hanyoyi guda biyu don shirya emulsions: wanda shine amfani da hanyar injiniyan don watsa mai ruwa a cikin ƙananan ruwa a cikin wani ruwa, wanda ake amfani dashi sosai a masana'antar shirya emulsions; Sauran shine a fasa ruwa a cikin wani yanki na kwayar halitta a cikin wani ruwa, sannan ka sanya shi tattara yadda yakamata a samar da emulsions.
Haske na emulsion shine ikon anti-barbashi wanda ke haifar da rabuwa da lokaci. Emulsions sune tsarin da ba zai dace ba tare da manyan kuzari kyauta. Sabili da haka, abin da ake kira emulsion ne a zahiri lokacin da ake buƙata don tsarin don rabuwa da daidaito ɗaya daga cikin ruwa a cikin tsarin don faruwa.
Lokacin da InterFachial Membrane tare da kitsen giya, mai acid mai yawa da kuma m kwayoyin da sauran kwayoyin halitta na Polar Organic, membrane ƙarfin mahimmanci mafi girma. Wannan saboda, a cikin adsorption adsorp of emulfier kwayoyin kwayoyin da kuma giya, acid, acid da Amines da sauran kwayoyin Powulcules don samar da "hadaddun", saboda haka membobin kwayar cutar, saboda haka, membrane membrane ta karu.
Emulsifers wanda ya ƙunshi Surfactants fiye da biyu ana kiransu gauraye emulsifiers. Gauraye emulsifier addorbed a ruwa / mai ke dubawa; Aikin intermololadular na iya samar da hadaddun. Saboda ƙarfin motsa jiki mai ƙarfi, tashin hankali yana raguwa sosai, adadin emulsifier addored a cikin keɓance yana ƙaruwa, ƙarfin nazarin ɓoyewar mahaifa yana ƙaruwa, ƙarfi yana ƙaruwa.
Cajin ruwan hawan ruwa yana da tasiri mai mahimmanci a kan kwanciyar hankali emulsion. Tabbatacce emulsions, wanda aka caje beads na ruwa wanda aka caje shi. Lokacin da aka yi amfani da ionic emulsifier, emulsifier IION adsorbed a cikin ke dubawa yana shigar da ƙungiyar Lipphilic da aka saka a cikin lokacin ruwa, don haka ya sanya ɗan gidan hydrophilic caads. Kamar yadda usullis beads da wannan caji, suna bin juna da sauƙin yin rauni, saboda haka ya karu. Ana iya ganin cewa mafi yawan emulsifier ions gargaded a kan beads, mafi girma cajin, mafi girman ikon hana beads daga agglomeration, da mafi barga da tsarin emulsion.
Haɗaɗɗen ƙwayar emulsion yana da wani tasiri a kan kwanciyar hankali emulsion. Gabaɗaya, mafi girman danko na matsakaici na watsawa, mafi girma kwanciyar hankali ga emulsion. Wannan saboda danko mai matsakaici ne babba, wanda ke da tasiri mai ƙarfi a kan motsi na ruwan hoda, saboda haka tsarin ya kasance mai barga. Yawancin lokaci, abubuwan polymer waɗanda za a iya narkar da su a emulsions na iya ƙara danko da kuma sanya kwanciyar hankali na emulsions mafi girma. Bugu da kari, polymers kuma iya samar da karfi membrane metfanes, yin tsarin emulsion ya tabbata.
A wasu halaye, kamar yadda foda mai ƙarfi zai iya sa emulsion yana haifar da tsinkaye. M foda yana cikin ruwa, mai, dangane da man, ruwa a kan ƙarfin mai ƙarfi, amma kuma rigar da mai, zai ci gaba da kasancewa a kan ruwa da ke dubawa.
Mai ƙarfi foda baya sanya emulsion barshi saboda foda ya shirya membrane mruscoles, don haka mafi kusantar kayan aikin foda, da mafi kusantar kayan jikin emulsion, da kuma barga da emulsion ne.
Surfacts suna da ikon ƙara yawan ƙwayoyin cuta ko abubuwa kaɗan mai narkewa bayan ƙirƙirar microoms bayani a cikin mafi yawan bayani, kuma mafita ba a wannan lokacin a wannan lokacin. Ana kiran wannan tasirin micelle. A Surfactant wanda zai iya samar da warware matsalar sofa, kuma kwayoyin halitta wanda aka kira slubilized kwayoyin da aka fi dacewa.

Kumfa yana taka muhimmiyar rawa a cikin Wanke. Kumfa shine tsarin watsawa wanda aka tarwatsa gas a cikin ruwa ko mai kauri, kamar yadda ake kiransa da ruwa mai laushi, kamar na filastik mai ruwa, gilashin fure, foamed ciminti da sauransu.
(1) samuwar kumfa
By kumfa muna nufin an tara kumfa iska ta rabu da membrane ruwa mai ruwa. Wannan nau'in kumfa koyaushe yana ƙaruwa da sauri zuwa babban ruwa saboda babban bambanci mai girma a cikin lokaci na tarawa (ruwa), haɗe tare da ƙarancin danko.
Tsarin samar da kumfa shine kawo adadin gas a cikin ruwa, kuma kumfa a cikin ruwa da sauri komawa zuwa ga karamin adadin gas mai ruwa.
Kumfa yana da mahimman halaye iri biyu dangane da ilimin halittar jiki: daya shine cewa kumfa ne mai rauni a cikin wani tsari, lokacin da ruwa fim din da aka watsa har zuwa wani lokaci, yana haifar da kumburin kumburi; Na biyu shine tsarkakakken ruwa ne ba zai iya samar da kumfa mai kyau ba, ruwa wanda zai iya samar da kumfa ne aƙalla biyu ko fiye. Mafita na ruwa na Surfactants sune tsarin tsarin da ke yiwuwa ga kumfa, da ƙarfin su na samar da kumfa kuma yana da alaƙa da sauran kaddarorin.
Surfacts tare da kyawawan ƙarfin foaming ana kiranta wakilan wasanni. Kodayake wakilin kumfa yana da ikon damfara mai kyau, amma coam da aka kirkira bazai iya kiyaye dogon lokaci ba, wannan shine, kwanciyar hankali ba lallai ba ne. Don kula da kwanciyar hankali na kumfa, sau da yawa a cikin mai ɗorewa don ƙara abubuwa waɗanda za su iya ɗaukar kayan shafa da kuma DocycyL Dimethylamilline.
(2) kwanciyar hankali na kumfa
Foam tsarin m tsarin da kuma yanayin karshe shi ne cewa jimlar farfajiyar ruwa a cikin tsarin yana raguwa bayan kumfa ya karye kuma yana raguwa da ƙarfi. Tsarin defloam ne tsari wanda ruwa mai ruwa ya raba gas ya zama mai kauri da bakin ciki har sai da ya karya. Saboda haka, digiri na kwanciyar hankali na kumfa shi ne yafi ƙaddara shi ne da saurin zubar da ruwa da ƙarfi fim na ruwa. Wadannan dalilai masu zuwa kuma suna tasiri wannan.
(3) hallaka
Asali na ka'idar kamun kumfa shine canza yanayin da ke samar da kumfa ko don kawar da abubuwan da suka haifar, don haka akwai hanyoyin da ke tattare da sinadarai da sunadarai.
Rashin lalacewa ta jiki yana nufin canza yanayin samarwa yayin riƙe da kayan sunadarai na kayan abinci, suna canzawa a cikin zafin jiki ko matsin lamba na zazzabi ko matsin lamba iri ɗaya ne na kawar da kumfa.
Hanyar sinadarai ta sinadarai ita ce ƙara wasu abubuwa don yin hulɗa tare da mai ɗorewa don rage ƙarfin damfara don haka ku sami manufar lalata, irin waɗannan abubuwan an kira su. Mafi yawan masu aikatawa sune surfaciants. Sabili da haka, gwargwadon tsarin defoaming, wanda aka ƙera ya kamata ya zama mai karfi da karfi don rage m tashin hankali, da kuma hulɗa tsakanin manyan kwayoyin a saman ƙasa yana da rauni, kuma hulda da adsorption kwayoyin da aka shirya a cikin sassauci.
Akwai nau'ikan defoamer daban-daban, amma m, duk su surfactantsan tsayayye ne. Non-Ionic Surfactants suna da kaddarorin anti-foaming na kusa ko sama da inda suka ga girgije kuma galibi ana amfani dasu azaman maganganu. Alburasa, musamman giya tare da sabon tsari, acid mai kitsen acid, polyamate mai, ana amfani da man silicone, da sauransu.
(4) kumfa da wanka
Babu hanyar haɗakar kai tsaye tsakanin kumfa da kuma wanka mai tasiri da kuma yawan kumfa baya nuna tasiri na wanke. Misali, baƙon da baƙon ba su da foaming kayan kwalliya fiye da soaps, amma maganin su sun fi kyau sabulu.
A wasu halaye, kumfa na iya taimakawa a cikin cire datti da fari. Misali, lokacin da wanke jita-jita a cikin gida, kumfa na abin sha ya ɗauki ɗigon mai kuma lokacin da keɓaɓɓen kifin, yana taimakawa ɗaukar ƙura, foda da sauran ƙazanta datti. Bugu da kari, wani lokaci ana iya amfani dashi azaman nuni ne game da ingancin abin wanka. Saboda mai mai kitse suna da kwaskwarima mai hana amfani da kayan abin wanka, lokacin da akwai mai yawa mai yawa, babu wani abin girbi mai yawa, babu coam mai yawa zai shuɗe ko na asali zai shuɗe. Hakanan ana iya amfani da kumfa a matsayin mai nuna alamar tsabta na kurkura, kamar yadda adadin kumfa a cikin kurkura bayani, don haka za'a iya amfani da yawan kumfa na rinsing.
A cikin babbar hankali, wanka shine aiwatar da cire abubuwan da ba'a so daga abin da za a wanke da kuma cimma wani dalili. Wanke a cikin tunanin da aka saba nufi da aiwatar da cire datti daga saman mai ɗaukar kaya. A wankewa, hulɗa tsakanin datti da mai ɗaukar nauyi ko kuma kawar da datti da mai ba da haske, kuma a ƙarshe datti ya rabu da mai ɗaukar kaya. Kamar yadda abubuwan da za a wanke da datti da za a cire su ne bambanci, wankewa shine tsari mai rikitarwa kuma ana iya bayyana ainihin hanyar Washin da ke cikin sauki a waɗannan abubuwa masu sauƙi.
Carraitialib Maza + Drorgent = Carrier + Dirt · Haskewa
Ana iya raba tsarin Wanke zuwa matakai biyu: Da fari dai, a ƙarƙashin aikin abin wanka, datti ya rabu da ɗaukarsa; Abu na biyu, datti na da aka watsa kuma an dakatar da shi a cikin matsakaici. Tsarin wankewa tsari ne mai annashuwa da datti kuma an dakatar da shi a cikin matsakaici na iya wanka. Saboda haka, mai ban tsoro mai ban tsoro ya kamata ya sami ikon watsa da dakatar da datti kuma hana ikon cire datti daga mai ɗaukar kaya.
(1) nau'ikan datti
Ko da don iri ɗaya, nau'in, abun da ke ciki da adadin datti na iya bambanta dangane da yanayin da ake amfani da shi. Droramar jikin mai shine yawancin wasu dabbobi da kayan lambu da ma'adinan ma'adinai (kamar ƙira, datti, datti, jini, jini, da sauransu.; Rtult daga abinci, kamar 'ya'yan itace stains, suna dafa mai staits, stailtim stread, sitaci, da sauransu.; Rantawa daga kayan kwalliya, kamar lipstick, goge na ƙusa, da sauransu.; Ranta daga yanayin, kamar soot, ƙura, laka, da dai sauransu.; Wasu, kamar tawada, shayi, shafi, da sauransu ya zo a cikin nau'ikan daban-daban.
Yawancin nau'ikan datti na iya rarrabu zuwa manyan rukuni uku: datti datti, datti ruwa da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da datti da na musamman da na musamman.
M datti m
Nain datti mai kauri ya haɗa da barbashi na ash, laka, ƙasa, tsattsarkan tsron baki. Yawancin waɗannan barbashi suna da cajin lantarki a farfajiyar su, yawancinsu ana cajin su sosai kuma ana iya magance su a sauƙaƙe a kan abubuwan fiber. M datti yana da wuya a narke cikin ruwa, amma ana iya tarwatsa shi da dakatar da mafita ta hanyar abin sha. M datti tare da karamin taro taro yafi wahalar cirewa.
② datti mai ruwa
Ruwa datti ne mafi yawan oil-mai narkewa, ciki har da man dabbobi, giya mai, mai da aka yi, mai da kuma fari. Daga cikin su, shuka da mai dabbobi, mai da alkali na alkalami, ba za'a iya saƙa a cikin giya, da kuma kayan ma'adinai maganin emulsification da watsawa. Draxuble ruwa datti yana da ƙarfi mai ƙarfi tare da abubuwan fiber, kuma ya fi ƙarfin adsorbed kan zaruruwa.
③ datti na musamman
Girman datti ya hada da sunadarai, sitaci, jini, fitsari, fitsari da ruwan 'ya'yan itace da ruwan' ya'yan itace. Yawancin irin wannan datti na iya cutar da su ta hanyar emolical da ƙarfi a kan abubuwan fiber. Saboda haka, yana da wuya a wanke.
Ba a samun nau'ikan datti shi kaɗai, amma galibi ana haɗuwa tare kuma ya ba da abu a kan abin. Horted na wani lokaci ana iya bayyana oxidized oxidized, bazu ko lalata a cikin tasirin tasirin waje, don haka ƙirƙirar sabon datti.
(2) Ingantaccen datti
Tufafi, hannaye da sauransu za'a iya lalata saboda akwai wasu nau'ikan hulɗa tsakanin abu da datti. Rantawa da abubuwa a cikin hanyoyi daban-daban, amma babu fiye da na jiki da sunadarai.
A ƙarfafawa, ƙura, laka, laka, yashi da gawayi zuwa sutura ne na zahiri. Gabaɗaya magana, ta wannan tsattsauran datti, da rawar da ke tsakanin abin da aka daidaita shi yana da rauni, cire datti kuma yana da daɗi. Dangane da runduna daban-daban, za a iya raba tsinkayen datti cikin miyar mashin da kuma ma'adinai.
A: Mashin mashin
Irin wannan tasirin zamani ya zama yana nufin adhesion wasu datti mai ƙira (misali, ƙura, laka da yashi). Ingantaccen injin na ɗaya daga cikin nau'ikan da aka warkarwa na m datti kuma ana iya cire shi da kyau na injina, amma lokacin da datti ya karami (<0.1um), ya fi wahala a cire.
B: Haɗin kai na lantarki
Mafi yawan tasirin wutan lantarki galibi suna bayyana a cikin aikin da aka caje su kan kayan datti a kan abin da akasari. Mafi yawan abubuwan fibrous ana tuhumar su sosai cikin ruwa kuma ana iya bin dattara cikin sauƙin da aka caji, kamar nau'in lemun tsami. Wasu datti, ko da yake an tuhumi marasa kyau, kamar su carbon baƙar fata, suna iya bin su gaba ɗaya a cikin ruwa (misali, Ca2, CA2, MG2 + da sauransu).
Hukumar lantarki ta fi ta ƙarfi sosai, yin datti da datti kuma da wahala.
② markarwa na sunadarai
Magani na sunadarai yana nufin sabon salon datti yana aiki akan abu ta hanyar sunadarai ko haɗin hydrogen. Misali, datti m datti, furotin, tsatsa da sauran m usds, giya da kuma wasu qungiyoyi masu sauki, giya mai sauki, giya mai sauki, giya mai sauki, giya mai kitse, giya mai sauki ne don samar da shaidu na ruwa. Sojojin sunadarai suna da ƙarfi da ƙarfi da datti saboda haka ya fi dacewa da abin da ya yi. Irin wannan datti yana da wuya a cire ta hanyar hanyoyin da aka saba kuma yana buƙatar hanyoyi na musamman don magance ta.
Digiri na Adshirin Adsharar datti yana da alaƙa da yanayin datti kanta da yanayin abin da aka saba. Gabaɗaya, barbashi a cikin sauƙi ga abubuwan fibaye. Karamin zabin da datti mai kauri, da karfi na m. Clarel datti a kan abubuwa masu ruwa kamar auduga da gilashin gilashi da gilashi sosai fiye da datti mara kyau. Rashin datti mara kyau yana da ƙarfi fiye da datti mai datti, kamar ƙura, ƙura da yumɓu, kuma ba shi da sauƙi don cirewa da tsabta.
(3) Maballin cirewar datti
Dalilin wanka shine cire datti. A cikin matsakaici na wani zazzabi (galibi ruwa). Yin amfani da tasirin jiki da sinadarai na kayan wanka don raunana ko kawar da sakamakon datti da kuma datti da kuma datti da kuma datti da wanke abubuwa daga dalilin lalata.
① Hanyar Cirewa Mai Girma
A: Wetting
Ruwan ruwa mafi yawa shine mafi yawan mai. Sawaran mai suna rigar mafi kyawun abubuwa kuma yada ƙari ko ƙasa da fim ɗin mai a saman fibrous kayan da ke saman fibrous kayan. Mataki na farko a cikin Wanke aikin shine rigar saman da ruwan wanke. Domin kare mai zane, saman fiber za a iya tunanin matsayin m m.
B: Digiri na mai - Curling Hanyar
Mataki na biyu a cikin Wanke na wanka shine cire mai da man shafawa, cire ruwa datti datti da aka samu ta wani irin mai shaye-shaye. Ruwa mai datti ya wanzu a farfajiya a cikin yada wani yaduwar man fim, kuma a ƙarƙashin tasirin rigar mai a saman hanya, wanda aka maye gurbinsu da wankan mai kuma daga baya aka maye gurbinsu a ƙarƙashin wasu sojojin waje.
② inji mai daskararru datti
Cire datti na ruwa shine mafi yawan abin da ya fi dacewa da daskararren datti ta hanyar iskar wanke, inda tsari na cirewa ya bambanta da rigar datti da mai ɗaukar maganin ta hanyar wanka. Saboda adsorption of Surfactants a kan m datti da mai ɗaukar hoto a tsakanin datti da kuma karfin da aka cire shi da sauƙin da datti kuma ana iya cire shi daga saman mai ɗaukar nauyi.
Bugu da kari, tallan surfactants, musamman wadatar zina, a kan farfajiya na datti da datti kuma mai ɗaukar hoto, wanda ya fi dacewa da cire datti. An tuhumi m samaniyoyi masu ƙarfi ko galibi ba daidai ba ne a caje su a cikin kafofin watsa labarai masu ruwa kuma na iya bamban da yadudduka na lantarki a kan talakawa ko kuma manyan yadudduka. Saboda tursuwar zargin zargin mai hade, mai samar da kayan datti a cikin ruwa zuwa ga daskararren farfajiya yana raunana. Lokacin da aka ƙara surfactant Surfactant, saboda zai iya daidaitawa a lokaci ɗaya mummunar ƙarfin damar rashin ƙarfi da haɓakar a tsakaninsu ya fi dacewa, kuma mai sauƙin cire shi ya fi sauƙi, kuma yana da sauƙi a cire shi.
Ba a tallata su ba gaba daya a kan gundumar manyan wurare kuma kodayake ba su canza yiwuwar hadewar adsorbed a farfajiya ba wanda zai taimaka wajen hana sake farfado da datti.
Game da batun Sadarwar Cationic, adsorptionsu na rage ko kawar da mummunar yanayin surface da kuma mai ɗaukar hoto, wanda yake rage haɓakar gtai da kuma saboda haka ba zai zama mai dacewa ga cire datti ba; Bugu da ƙari, bayan adsorption a kan m farfajiya, cationic Surfactants suna juya m surfrophobic freading surferhobic fru surfrophobic freading surfrophobic freading surfrophobic surferhobic surferhobic surferhobic surferhobic surferhobic surferhobic surferhobic surferphobobic kuma saboda haka ba zai iya samar da jingina ba.
③ cire na musamman ƙasa
Protein, sitaci, dan adam asarar dan adam, ruwan 'ya'yan itace shayi da sauran irin wannan datti suna da wahala su cire tare da surfactants na al'ada kuma suna buƙatar jiyya na musamman kuma suna buƙatar jiyya na musamman.
Abubuwan sunadari kamar su kamar cream, ƙwai, jini, jini, madara da kuma irin fata da ke rufe kan zaruruwa. Za a iya cire kayan kare kariya ta hanyar amfani da gamsarwa. Kayayyakin enzyme ya karya sunadarai a cikin datti a cikin amino acid ruwa ko OliigoPepties.
Stoach stain zo daga kayan abinci, wasu kamar gravy, manne da tasirin catalytic a kan hydrolysis na sitaci m, yana haifar da sitaci don karya sugabs.
Lipase Catalyzes The Triglycosistides, wanda ke da wuya a cire ta hanyar al'ada, kamar sebum da mai cin abinci, kuma ya karya su cikin narkewa glycle da mai acid.
Wasu yankuna masu launin cike da ruwan 'ya'yan itace, ruwan' ya'yan itace shayi, inks, lipstick da sauransu galibi suna da wuyar tsabta sosai ko da bayan maimaita wankewa. Wadannan rigunan ana iya cire su ta hanyar yin amfani da oxridiz ko kuma rage kayan aiki kamar suleach, wanda ke lalata su cikin ƙananan kayan aiki mai launi.
(4) Cikanci na cire tsarin tsabtatawa
Abubuwan da ke sama shine ainihin ruwa a matsayin matsakaici na wankewa. A zahiri, saboda nau'ikan sutura da tsari, wasu sutura suna amfani da wanke ruwa da maɗaukaki da maɗaukaki da maɗaukaki da maɗaukaki, da kuma bushe da sauƙi don girgiza kai, saboda haka bayan wankewar zai mutu; Ta hanyar ulu Samfuran sutthun kuma sau da yawa suna fitowa sau da yawa ana bayyana shrinkage, wasu samfuran Woolen tare da wankan ruwa kuma yana da sauƙin kwantar da hankali, canjin launi; Wasu siliki hannun siliki suna yin watsi da su bayan wanke da rasa luster. Ga waɗannan tufafin sau da yawa suna amfani da hanyar bushe-tsaftacewa don lalata. Abin da ake kira tsabtatawa bushe yana nufin hanyar wankin a cikin kwayoyin cuta, musamman a cikin abubuwan da ba su da ruwa.
Dry tsabtacewa shine nau'in mai wanka da wanka. Saboda tsabtataccen tsabtatawa baya buƙatar lalacewa sosai, ba ta haifar da lalacewa sosai, wrinkling da nakasa ga sutura, yayin da wakilai na tsabtatawa suka bushe, ba lallai ba ne ruwa, da wuya fitar da fadada da ƙanƙancewa. Muddin fasaha ana kulawa da kyau, ana iya tsabtace tufafin da tsabtace ba tare da murdiya ba, launi fadada da tsawaita rayuwar sabis.
Dangane da tsabtataccen tsabtatawa, akwai nau'ikan datti guda uku.
Dirlum mai narkewa datti ya hada da kowane irin mai da man shafawa, wanda yake shine ruwa ko mai laushi kuma za'a iya narkar da shi a cikin tsabtataccen tsaftacewa.
Lentadil-mai narkewa datti mai narkewa yana narkewa a cikin mafita na ruwa, amma ba a cikin wakilan tsabtatawa ba, kamar silnic, silnic, sitaci, furotin, da sauransu.
M da ruwa wanda shigar datti mai narkewa ba mai narkewa a cikin ruwa ba, kamar oxbon daban-daban da ankara, da sauransu.
Saboda yanayin daban-daban na nau'ikan datti, akwai hanyoyi daban-daban na cire datti a cikin tsarin bushe-tsaftacewa. Man mai narkewa, kamar dabbobi da man kayan lambu, man marks da man shafawa, suna sauƙin narkewa a cikin abubuwan da aka daskarewa kuma za'a iya cire shi sau da sauƙi a cikin tsabtatawa. Madalla da narkewa na daskararre-tsaftacewa na mai da man shafawa da gaske ya fito ne daga sojojin Van der Der Gallan Pan Der Der Der Bonts.
Don cire datti mai narkewa kamar inorganic, sugars, sunadarai da gumi, ƙazanta mai narkewa yana da wuya a cire shi daga sutura. Koyaya, ruwa yana da wuya a soke a cikin wakilin bushe-tsaftacewa, saboda haka don ƙara yawan ruwa, kuna buƙatar ƙara surfacits. Kasancewar ruwa a cikin wakilin bushe-tsaftacewa na iya sanya farfajiya na datti da sutura ta lalace, wanda yake da sauki a yi hulɗa da gungun polar, wanda yake da mai dacewa ga adsorction na Surfactants a farfajiya. Bugu da kari, lokacin da Surfactants siffofin miceles, datti mai narkewa da ruwa za a iya yin narkewa a cikin micelles. Baya ga kara abun ciki na ruwa na daskararren-tsaftacewa, surfactants na iya taka rawa wajen hana sake fasalin datti don inganta tasirin lalata.
Kasancewar karamin adadin ruwa wajibi ne don cire datti mai santsi, amma ruwan da yawa na iya haifar da murdiya da wrinkling a cikin wani suttura-tsaftacewa dole ne ya zama matsakaici.
Rnil wanda ba ruwa-narkewa ko mai narkewa, barbashi mai ƙarfi kamar ash, laka, ƙasa da carbon baƙar fata, an haɗa shi da mai. A cikin tsabtatawa bushe, kwararar sauran ƙarfi, tasiri na iya yin tallan datti da datti kuma wanda aka haɗa da masu tsaftataccen ƙwayar ƙasa, watsawa, don hana sake sakawa a gaban sutura.
(5) Abubuwa 5 da suka shafi aikin wanka
Adadin Tsarin Surfactant na Surfactants a cikin ke dubawa da rage tashin hankali (Interfacial) tashin hankali ne a cikin cire ruwa ko datti datti. Koyaya, tsarin wankin yana da hadaddun da kuma wanke sakamako, har ma da nau'in shago iri ɗaya, yana rinjayi wasu dalilai da yawa. Waɗannan abubuwan sun haɗa da taro na abin wanka, zazzabi, yanayin yanayin abin da aka yi, nau'in fiber da tsarin masana'anta.
① Surfactant maida hankali
Mulakalin Surfactants a cikin Magani taka muhimmiyar rawa a cikin Washe tsari. Lokacin da maida hankali ya kai ga maida hankali kanzarin micelle (CMC), sakamako na wanke yana ƙaruwa sosai. Sabili da haka, taro na wanka a cikin sauran ƙarfi ya zama ya fi darajar CMC don samun sakamako mai kyau. Koyaya, lokacin da hankali na surfactant ya fi darajar CMC, haɓaka haɓaka a cikin wankewar wanka ba a bayyane yake ba kuma ba lallai ba ne don ƙara yawan ɗaukar fansa da yawa.
Lokacin da cire mai ta hanyar narkewa, ingantaccen sakamako yana ƙaruwa tare da ƙara yawan taro, koda lokacin da aka gabatar da maida hankali ya wuce CMC. A wannan lokacin, yana da kyau a yi amfani da kayan wanka a cikin yanayin tsakiyar yankin. Misali, idan akwai datti da yawa a kan cuffs da abin wuya na sutura, ana iya amfani da wani yanki na abin wanka don ƙara yawan narkar da surfactant akan mai.
②etempeates yana da tasiri mai mahimmanci akan aikin lalata. Gabaɗaya, ƙara yawan zafin jiki yana sauƙaƙe cire datti, amma wani lokacin maɗau maɗa yana da girma kuma zai iya haifar da rashin nasara.
Theara yawan zafin jiki yana sauƙaƙe wanda ya yuwama, mai sauƙi man shafawa a yanayin zafi sama da karuwa yana ƙaruwa cikin kumburi saboda karuwa a cikin zazzabi, duk wanda sauƙaƙe cire datti. Koyaya, don daidaitawa, microgps tsakanin zaruruwa suna raguwa kamar yadda 'yan fashi suka faɗo, wanda yake shine rashin lahani ga cire datti.
Canje-canje na zazzabi kuma suna shafar ƙima, CMC darajar da girman micelle na surfactants, don haka ya shafi iskar wanke. Rashin daidaituwa na Surfactants tare da dogon carbon sarƙoƙi ya ragu a ƙananan yanayin zafi kuma wani lokacin da za a tashe darajar CMC, saboda haka wankewar zafi ya kamata a tayar da shi ta dace. Tasirin zazzabi a kan darajar CMC da girman micelle ya bambanta ga ionic da ba ionic Surfacts. Don ionic surfactants, karuwa a cikin zazzabi gabaɗaya yana ƙaruwa da ƙimar CMC kuma yana rage girman micelle, wanda ke nufin cewa maida hankali ne ga Surfactant a cikin isasshen bayani ya kamata a ƙara ƙaruwa. Ga surfactants mara kyau, karuwa a cikin zazzabi yana haifar da raguwa a darajar CMC da kuma haɓaka haɓakar micelle, don haka a bayyane yake cewa haɓakar ƙwayoyin cuta zai taimaka tasirin da ba shi da aiki. Koyaya, zazzabi kada ya wuce wurin girgije.
A takaice, mafi kyawun wanke zafin jiki ya dogara da kayan wanka da abun da ake wanke. Wasu kayan wanka suna da tasirin girbi a zazzabi a ɗakin, yayin da wasu suna da sabani daban-daban tsakanin wanka mai sanyi da zafi.
Foam
Yana da al'ada don rikitar da ƙarfin fewing tare da Wanke da Wanke, gaskata cewa kayan wanka da ƙarfi mai ban sha'awa suna da sakamako mai kyau. Bincike ya nuna cewa babu dangantakar kai tsaye tsakanin tasirin wanke da kuma yawan kumfa. Misali, wanke tare da mawuyacin kayan wanka ba shi da tasiri fiye da wanka tare da kayan girbi na boaming.
Kodayake kumfa ba da alaƙa da wankewa ba, akwai lokatai idan ya taimaka wajen cire datti, alal misali, lokacin da wanke kwano da hannu. Lokacin da goge kera, kumfa kuma zai iya kawar da ƙura da sauran m barbashi, asusun.
Powerarfin Foaming kuma yana da mahimmanci ga shamfu, inda kyakkyawan kumfa ya haifar da ruwa mai kyau a lokacin shamfu ko wankin yana barin gashin gashi mai mai da kwanciyar hankali.
④ nau'ikan zaruruwa da kaddarorin jiki na tothales
Baya ga sinadaran sunadarai na zaruruwa na zaruruwa, wanda ke shafar tasirin matsakaicin da kuma fitar da ƙabilu da kuma ƙungiyar 'yan fashi da sulhu suna da tasiri kan sauƙin cirewa.
Sikeli na ulu zaruruwa da kuma rabban lebur mai lankwasa na fibers na auduga sun fi yiwuwa tara datti fiye da ƙwararrun zaruruwa. Misali, Carbon baki cike da fina-finai na selulo (propcose fina-finai) yana da sauƙin cirewa, yayin da carbon baki stained akan kayan auduga yana da wuya a wanke. Wani misali kuma shi ne cewa ƙashin masana'anta na fiber da aka yi da polyester sun fi yiwuwa su iya yiwuwa su cire abubuwan mai na fiber a kan yadudduka mai.
Tighly twisted yarns da m yadudduka, saboda karamar rata tsakanin zaruruwa na datti, amma da zarar wanke wanke shi kuma ya fi wahala.
⑤ Hardning na ruwa
A maida hankali ne na CA2 +, MG2 + da sauran ions na karfe a cikin ruwa suna da babban tasiri a kan wanka, musamman lokacin da anions samar da alli da kuma silseum silse wanda yake ƙasa da narkewa kuma zai rage hani hebrency. A cikin ruwa mai wuya, koda da maida hankali ne na surfactant yana da yawa, abin hucin gwiwar har yanzu ya fi muni da distillation. Don Surfactant don samun mafi kyawun isasshen wanka, taro na ca2 + ions a cikin ruwa ya kamata a rage 1 x 10-6 Mol / mg3 zuwa 0.1 MG / L) ko ƙasa da ƙasa. Wannan yana buƙatar ƙarin ƙari na mayukan mai cike da abin sha.
Lokaci: Feb-25-2022



