Teburin abinda ke ciki na wannan labarin:
1. Ci gaban amino acid
2. Cikakken kaddarorin
3. Abubuwan sunadarai
4.Classification
5. Kirais
6. Phychicochemical kadarorin
7. Mai wahala
8. Ayyukan Antificrobial
9. Kayayyakin mallaka
10. Aikace-aikace a cikin masana'antar Cosmetic
11. Aikace-aikace a cikin kayan kwalliya na yau da kullun
Amino acid Surfactants (Aas)sune aji na Surfactants wanda aka kafa ta hanyar hada kungiyoyin hydrophobic tare da hade daya ko fiye da amino acid. A wannan yanayin, amino acid din na iya zama roba ko da aka samo daga furotin hydrolysates ko makamancin haka. Wannan takarda ta rufe cikakkun bayanai game da yawancin hanyoyin roba don AAS kuma tasirin hanyoyi daban-daban akan physicochorthals na ƙarshe samfuran, watsawa da kuma socictionila da kuma ƙwayoyin cuta. A matsayinka na aji na surfactants a cikin kara bukatar, da ayoyinsu na AAS saboda yanayin canji yana ba da adadin damar kasuwanci.
Ganin cewa ana amfani da Surfactants ana amfani dashi sosai a cikin wanka, emulsifiers, morrosion mai da aka ba da izini, masu bincike ba su daina kula da surfactant ba.
Surfacts sune yawancin samfuran sinadarai waɗanda ke cinye su a adadi mai yawa a kan yau da kullun a duniya kuma sun sami mummunar tasiri a cikin yanayin ruwa.Karatun ya nuna cewa yawan amfani da gargajiya na kayan gargajiya na iya haifar da mummunar tasiri a kan yanayin.
A yau, ba mai guba ba, tsirara da biocompacizawa suna kusan mahimmanci ga masu sayen mutane kamar yadda amfani da aikin Surfactants.
Bioosurfactantsan wasan kwaikwayo mai ɗorewa shine wanda ƙananan halittar ƙwayoyin cuta ke haifar da ƙwayoyin cuta, fungi, da yisti, ko kuma yisti.Sabili da haka, BIOSURFACTING.
Amino acid Surfactants (Aas)suna ɗaya daga cikin abubuwan da ake amfani da su, yawanci ana samarwa daga dabba ko aikin gona da aka samo kayan ƙasa. A cikin shekaru 20 da suka gabata, Aas sun jawo hankali sosai daga masana kimiyya a matsayin Surfacts a cikin sabuntawa, amma kuma saboda AAS suna iya zama daɗaɗa abubuwa da yawa kuma suna da aminci ga yanayin.
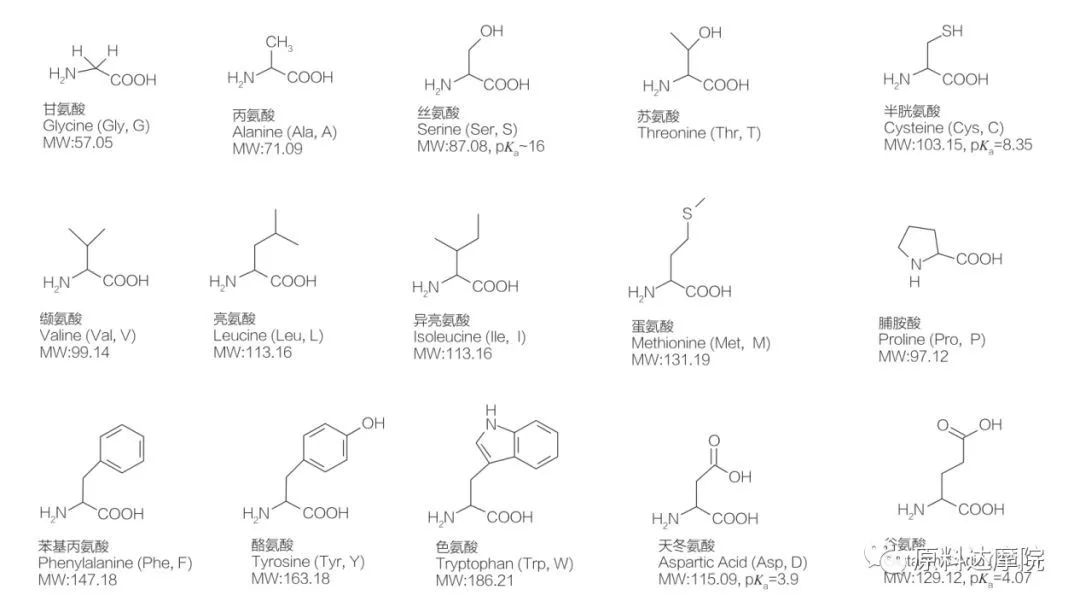
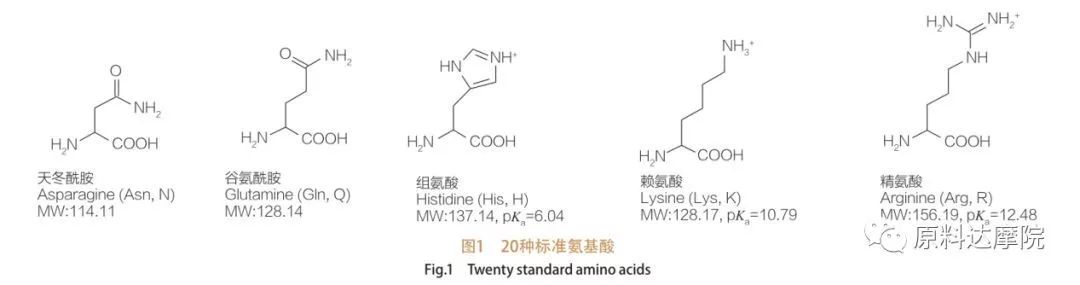
AAS za a iya bayyana a matsayin aji na surfactants wanda ya ƙunshi ƙungiyoyin amino acid (HO 2 C-Chr-NH 2) ko ɓarna na AT 2 (HA 2 C-Chr-Nh-). Yankin yankuna na 2 na amino acid sun ba da damar haifar da ƙwararrun surfactants da yawa. A total na 20 na daidaitattun amino acid 20 an san su wanzu cikin yanayi kuma suna da alhakin duk halayen da aka yi a cikin ayyukan rayuwa da rayuwa. Sun bambanta da juna kawai bisa ga ragowar r (Hoto na 1, PK A ne mummunan logarithm na acid na rashin tabbas. Wasu ba su da-polar da Hydrophobic, wasu sune polar da Hydrophilic, wasu sune ainihin kuma wasu sune acidic.
Saboda amino acid abubuwa ne mai sabuntawa, surfactantsan da ke hade da amino acid masu suna da babban damar yin dorewa da tsabtace muhalli. Tsarin sauki da na halitta, ƙananan guba da sauri da sauri sau da yawa suna sa su fifisu su fi dacewa da surfactants na al'ada. Ta amfani da kayan albarkatun ƙasa (misali amino acid da man kayan lambu), AAS za a iya samar da AAS daban-daban da hanyoyin sunadarai daban-daban.
A farkon karni na 20, an gano amino acid don substrates don setthesis na surfactants.An yi amfani da Aas galibi azaman abubuwan adawar a cikin magunguna da kayan shafawa.Bugu da kari, an gano AAS ta zama mai aiki da kwayoyin halitta a kan cututtukan cututtukan cututtukan cuta, ciwace-ciwacen cuta, da ƙwayoyin cuta. A cikin 1988, kasancewar Aas Aas Aas Aas an samar da sha'awar bincike a cikin aiki. A yau, tare da ci gaban ilimin ilimin kimiyyar kereetchnold, wasu amino acid din suna iya haifar da kasuwanci a kan babban sikeli da yisti, wanda ke tabbatar da cewa samar da Aas yana tabbatar da cewa samar da AAS yana da abokantaka.
01 ci gaba da amino acid
Tun da wuri a farkon karni na 19, lokacin da aka gano tsarin amino acid, an annabta tsarinsu don su kasance mai mahimmanci - ana amfani dasu azaman kayan amfanin gona don shiri na ishiphiphaimes. Na farko na binciken a kan tsarin Aas an ruwaito ta hanyarsa a 1909.
A cikin wannan nazarin, N-Acylglycine da N-Acylalla an gabatar da su a matsayin kungiyoyin hydrophilic don surfactants. Aikin mai zuwa ya shafi tsarin Liboamino acid (AAS) ta amfani da Glycine da Alasine, da Hentrich et al. buga jerin abubuwan binciken,Ciki har da aikace-aikacen lambun farko, kan yin amfani da acyl sarcarate da acyl asirin salts kamar yadda Surfactants a cikin kayan tsabtace gida (misali shamfu, kayan abinci da haƙoran haƙori).Bayan haka, yawancin masu bincike da yawa sun bincika tsarin kira da kuma Phyicochemical Prive na Acyl Amino acid. Zuwa yau, an buga babban gungumiyar littattafai a kan synthesis, kayan aiki, aikace-aikacen masana'antu da kuma biodorad ga Aas.
02 Cikakken Cikakken
Wanda ba na Polar ba na Aas na Aas na iya bambanta cikin tsari, tsayin sarkar da lamba.Tsarin tsari da kuma ayyukan farfajiya na Aas suna bayyana bambance-bambancen halayensu da na ilimin kimiyyar ilimin kimiyyar halitta. Kungiyoyin kai na Aas sun hada da amino acid su ko peptides. Bambanci a cikin kungiyoyin kai suna tantance adsorption, tarawa da ayyukan halittu na wadannan surfactants. Kungiyoyin aiki a cikin shugaban kungiyar sannan kuma sanin irin Aas, gami da Cationic, anionic, ba da son rai ba. Haɗin Hydrophilic amino acid da tsayin daka tsintsiya tsarin tsari yana samar da tsarin amphilic wanda ke sa kwayoyin daɗaɗɗar kwayar cutar aiki. Bugu da kari, gaban ASYMMetric Carbon atoms a cikin kwayoyin halittar yana taimakawa samar da kwayoyin kamuwa da kamal mai.
03 Compossion Composition
Duk peptides da polypeptides samfuran polymerization na waɗannan kusan 20 α-amino acid. Dukkanin 20 α-amino acid sun ƙunshi ƙungiyar Mataxylic acid (-Cooh) da ƙungiyar masu amfani (-NH 2), duka biyu sun haɗe zuwa wannan Teetran Terom. Amino acid ya bambanta da juna ta hanyar ƙungiyoyi daban-daban na r a haɗe zuwa α-carbon (amma na lycine, girma da caji (acid, alkaliniti). Waɗannan bambance-bambance kuma suna ƙaddara soci acid ruwa cikin ruwa.
Amino acid sune wasan kwaikwayo (ban da glycine) kuma suna da aiki mai kyau ta hanyar yanayi daban-daban da ke da alaƙa da Alpha Carbon. Amino acid suna da abubuwa biyu masu yiwu; Ba su da hotunan madubi na juna da na juna, duk da cewa yawan L-siteroisomers yana da matukar muhimmanci. R-rukuni na yanzu a wasu amino acid (pheranlaline da Tastrophan) shine Aryl, yana haifar da iyakar karin UV sau 280 a raga. A +-cooh da muhimmin α-NH 2 a cikin amino acid suna da ikon ionization, kuma duka setereoisomers da aka nuna a ƙasa.
R-Cooh err-Coo-+ H+
R-nh3+↔-nh2+ H+
Kamar yadda aka nuna a cikin ionization daidaito a sama, amino acid sun ƙunshi aƙalla biyu marasa lafiya ƙungiyoyi; Koyaya, ƙungiyar Carboxyl ta fi kyau a acidic sosai idan aka kwatanta da ƙungiyar amino. PH 7.4, an hana ƙungiyar CARBOBOLYL yayin da aka sanya kungiyar Amino ta nuna. Amino acid tare da r under-ionizable r nemral a wannan ph kuma samar da zwitterion.
04 Classigfication
Aas ana iya rarrabe su bisa ga ka'idoji hudu, wanda aka bayyana a ƙasa bi da bi.
4.1 A cewar asalin
| A cewar asalin, ana iya raba AAS zuwa rukuni guda 2 kamar haka. ① na halitta Wasu halaye na zahiri dauke da amino acid sunada amino acid suna da ikon rage girman kan sama / intfacial, kuma wasu ma sun zartar da ingancin glycolipids. Wadannan Aas an san shi da lippeptides. Liperopepides ne ƙananan ƙananan ƙwayoyin cuta mai nauyi, yawanci ana samarwa ta jinsunan bacillus.
Irin wannan Aas an kara raba kashi uku:Surfactin, Iturin da Fengycin.
|
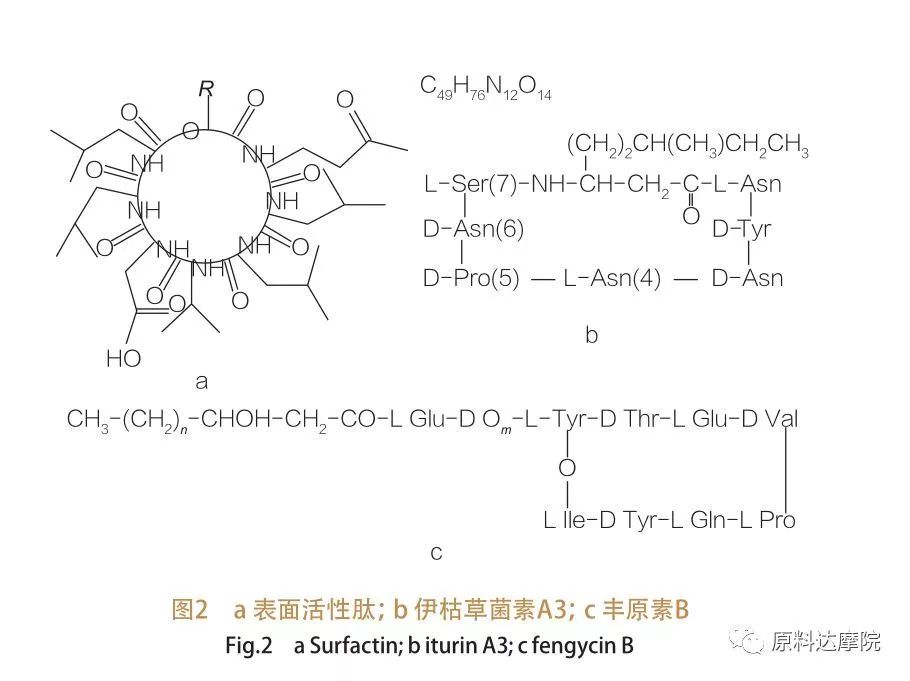
| Iyalin Peptides na farfajiya na Kasa sun ƙunshi bambance bambancen Heptpeptipp na abubuwa daban-daban,Kamar yadda aka nuna a cikin Hoto na 2a, wanda C12-C16 wanda ba a tantance β-hydroxy mai kitse na β-hydroxy sarkar da aka haɗa da peptide. A farfajiya peptide lactone lacone ne wanda aka rufe ta catalysis ya rufe tsakanin C-βU na β-hydroxy kitse da peptide. A cikin Subclass na ITUUurin, akwai manyan bambance-bambance-bambance-bambance, wato Iturin a da c, mycosubtilin da Bchilliscin D, F da L.A duk al'amuran, ana danganta da sarƙoƙi na C14-C17 na β-amino mai guba na β-aminiya acid (sarƙoƙi na iya zama dabam-dabam). Game da batun Ekurimycys, da Amino Rukunin kan β-matsayi zai iya samar da aminin kai tare da C-Terminus Ta haka ne samar da tsarin lactam.
The Subclass Fengycin ya ƙunshi fengyncin A da B, wanda kuma ana kiranta Plalipastatin yayin Tyr9 an saita shi.Hawan ragi yana da alaƙa da C14 -C18 cikakke ko kuma ba a tantance Β-hydroxy sarkar kitse. Plepastatin shima lactone lactone ne, dauke da sarkar gefen tyr a matsayi na 3 na peptide jerin abubuwan ciki, don haka ne samar da tsarin zobe na ciki (kamar yadda lamari ne na zoben zobe na ciki (kamar yadda lamari ne na zoben zobe na ciki (kamar yadda lamari ne na zoben zobe na ciki (kamar yadda lamari ne da yawa daga cikin liudomonas lipopedees).
Kasar roba AAS kuma zai iya amfani da ta amfani da kowane acidic, asali da tsakaitaccen amino acid. Amino acid gama gari da aka yi amfani da shi don tsarin AAS suna da glutamic acid, maci, ciyawar, da hlycine, da hydrolysates. Wannan subclass na Surfactants na iya shirya ta hanyar sinadarai, enzymatic, da kuma sinad da suke chemoenzymatic; Koyaya, don samar da Aas, gyaran sinadarai shine mafi wadatar tattalin arziki. Misalai gama gari sun hada da N-Lauroyl-L-Parutamic acid da n-parmoyl-l-glutamic acid.
|
4.2 dangane da sarkar sarkar alifatic
Dangane da sarkar sarkar alippatic, za a iya raba Surfactants na tushen amino acid zuwa nau'ikan 2.
Gwargwadon matsayin mai canzawa
| ①n-maye gurbin Aas A cikin mahimmin mahadi, ana maye gurbin rukunin amino ko kungiyar mai kula da carbophyl, sakamakon asarar asali. Mafi sauki misalin n-setited Aas ne n-acionic amino acid ne, waɗanda suke ainihin surfactant surionic surfactant. N-Subyited Aas suna da haɗin haɗin kai tsakanin hydrophobic da hydrophilic rabo. Amide Bond yana da ikon samar da haɗin hydrogen, wanda ya sauƙaƙe lalata wannan surfactant a cikin yanayin acidic.
②c-maye gurbin Aas A cikin C-Subtuted mahadi, da musayar ya faru ne a rukunin Carboxyl (ta hanyar zama ko ester bond). Na yau da kullun C-subituted mahadi (misali esters ko kuma a amdes) sune Suri na Cayinic.
③n- da C-maye gurbin Aas A cikin irin wannan surfactant, duka amino da ƙungiyoyin carbozyl sune ɓangare na hydrophilic. Wannan nau'in shine ainihin surfactant. |
4.3 bisa ga adadin wutsiyoyi na hydrophobic
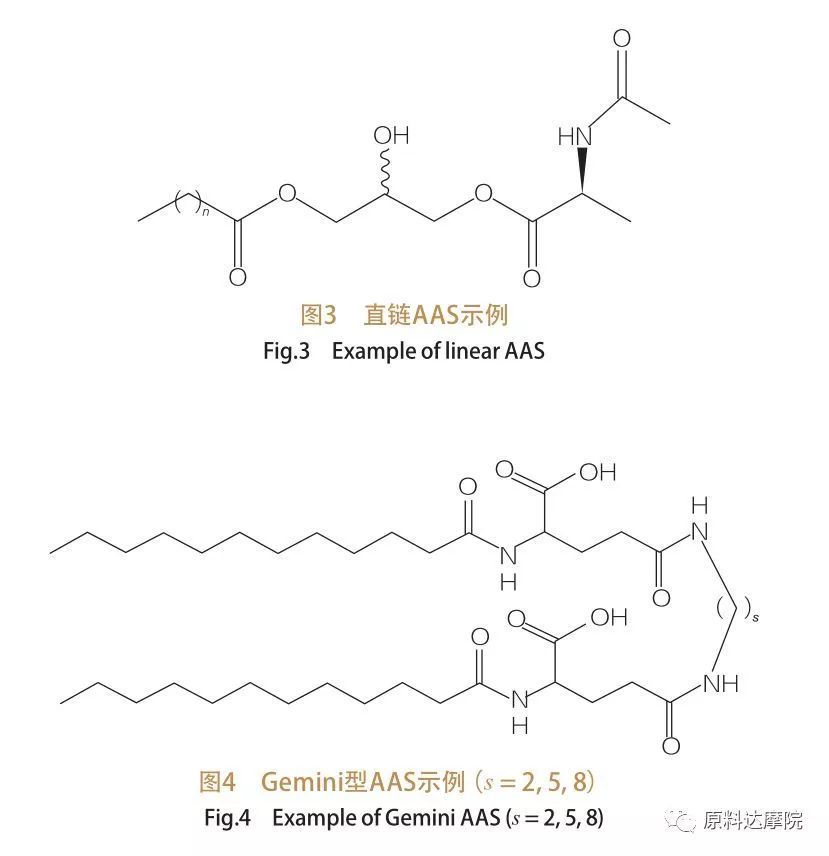
Dangane da yawan adadin kai da wutsiyoyi na hydrophobic, ana iya raba su zuwa rukuni hudu. Madaidaiciya-sarkar Aas, Gemine (Dimer) Type type Aas, glycolerrollid nau'in Aas, da kuma ampphanid Typeichilic (BOLA) Type Aas. Surfacts madaidaiciya shine Surfacts wanda ya kunshi amino acid da wutsiya na hydrophobic guda ɗaya (Hoto 3). Gemini Nauga Aas suna da amino acid shugaban gida biyu da kuma wutsiyoyi biyu na ruwa a kowane kwayar halitta (Hoto na 4). A cikin wannan nau'in tsari, madaidaiciya-sarkar aas suna da alaƙa da wani sarari kuma saboda haka kuma ana kiranta dimers. A cikin nau'in glycerollidodi Aas, a gefe guda, wutsiyoyi biyu na hydrophobic sun haɗe zuwa ƙungiyar shugaban amino acid. Wadannan jerin surfactants za a iya ɗauka azaman analogly na Monoglyceries, diglerides da phospholipids, yayin da a Boospholipids na Hydrophobic Wutsiya na Hydrophobic.
4.4 A cewar nau'in kungiyar kai
①cationic aas
Shugaban kungiyar irin wannan nau'in surfactant yana da babban caji. Aas na farko na Cayintiic Aas shine ethyl Cocoyl arginate, wanda shine Carboloyone Carboxylate. Ka'idodin na musamman da daban-daban na wannan surfactant sa shi da amfani a cikin masu shan hankali, wakilan antimicrobial, wakilai na antimatic, da kuma mai saukin kai a kan idanu da fata da sauri. Yin ringare da mhatre setnthesized arginine-tushen hadewar ciminic aas kuma kimanta abubuwan da aka kimanta su na likitoci. A cikin wannan binciken, sun da'awar babban da ake samu na samfuran da aka samu ta amfani da yanayin da baumann yanayin. Tare da kara tsawan alkyll na alkyll da hydrophobicity an gano shi don ƙaruwa da mahimmancin ƙwayoyin cuta (CMC) don raguwa. Wani kuma shine furotin ACYL, wanda ake amfani dashi azaman kwandishan a samfuran kiwon gashi.
②anionic aas
A cikin surfactants na anionic, polar shugaban kungiyar Surfactant yana da mummunan caji. Sarcosine (ch 3 -nh-ch 2 -Cooh, n-methylglycine, wani amino acid din da aka samo a cikin Urchine da Bahar Amino acid da aka samo a cikin sel na dabbobi. -Cooh,) shine mai alaƙa da Glycine, wanda shine amino acid na asali a cikin sel na dabbobi. Lauric acid, Tetrownoic acid, acid acid da heliince da aka saba amfani da su don amfani da sentuctant sarcosate Surcactants. Sarcarates suna da m ne kuma ana amfani da shi a cikin bakin shafe, shamfu, masu tsabtace fata, da sauran samfuran kwaskwarima.
Sauran ayyukan anionic na samuwa sun hada da Amsoft CS-22 da Amilitegck-12, waɗanda suke suna na Kasuwanci don Sodium n-Grutam-L-ccoylate da potassium n-cocoyl glycate, bi da bi. Ana amfani da Amilite azaman mai ban sha'awa wakili, kayan wanka, mai narkewa, kayan wanki, masu tsabtace fuska, suna tsaftace su da Surfacts suna tsaftace su da Surfacts Fuskants. Ana amfani da Amisoft a matsayin mai laushi mai laushi da tsabtace gashi, galibi cikin abubuwan tsarkakewa, samfuran kula da jiki, shamfu da sauran samfuran kulawa na fata.
③zwitterionic ko amphoteric aas
Amphoteric Surfactants sun ƙunshi wuraren acidic da na yau da kullun kuma na iya sauya cajin su ta hanyar canza darajar PH. A cikin kafofin watsa labarai masu jarida suna nuna hali kamar suractantsan acionic, yayin da cikin yanayin acidic da suke nuna hali kamar Surfactants na tsaka-tsaki da tsaka tsaki da tsaka-tsaki. Luryal Lysine (ll) da alkoxy (2-hydroxypropyl) arginine ne da aka san Surfacts Surfactants dangane da amino acid. Ll samfurin inganin lysine da lauric acid. Saboda tsarinsa na sihiri, ll yana da banƙyama a kusan duk nau'ikan gashin girki, ban da alkaline sosai ko kayan kwalliya sosai. A matsayinar da foda na kwayoyin, ll yana da kyakkyawan taso a cikin kayan masarufi da ƙananan ƙwayoyin cuta, ba da wannan surfactic ikon. Ana amfani da ll sosai a cikin cream na fata da kuma kwandishan gashi, kuma ana amfani dashi azaman mai tsami.
④nononiic aas
Baƙon da ba a san su ba ne ta hanyar rukunin Shugabannin Polar ba tare da caji ba. TAMBAYA TAMBAYA TAFIYA DA AL-SABAGH et al. daga mai-zame α-amino acid. A cikin wannan tsari, L-Phenylalaine (lep) da L-leucine sun haɗu da HexadeCancanol, suna biye da shi tare da palmitic acid don acid na palmedicic da ashin dabbobi biyu na α-amino acid. Amidesin da Esters sannan suka karyata halayen ingancin ethylene da oxide uku tare da lambobin daban-daban na Polyoxyethylene (40, 60 da 100). An gano waɗannan Aas ɗin ba da ba su da kyakkyawar rigakafin da kayan kwalliya.
05 kira
5.1 Hanya na yau da kullun
A cikin Aas, ƙungiyoyin Hydrophobic da aka haɗe za a iya haɗe zuwa Amine ko rukunin gidan carboxylic acid, ko ta hanyar sarƙoƙin amino acid. Dangane da wannan, ana samun hanyoyin roba na asali huɗu, kamar yadda aka nuna a hoto na 5.
FIG.5 Hanyoyin Synthesis
| Hanya 1. Amigphilic Ester Amines an samar da su ta hanyar halayen canji, a cikin wane yanayi mai ɗaukar nauyi ne wanda yakan sami kitfact ɗin giya da amino acid ɗin da mai kara kuzari. A wasu halayen, ayyukan sulfuri acid kamar yadda duka mai kara kuzari da wakili mai narkewa.
Hanyoyi 2. A kunna amino acid amsa tare da alkylamines don samar da shaidu na Amide, wanda ya haifar da tsarin kira na amippilic amdoamines.
Hanya 3. Amido acid ne ya hade ta hanyar amsawa kungiyar amino acid tare da Amido acid.
Hanyoyi 4. Duk da haka-sarkar Alkyl da aka dauki ta hanyar amsawa da kungiyoyi na yau da kullun. |
5.2 Ci gaban a cikin kira da samarwa
5.2.1 Hythilesis na sarkar amino acid / peptide surfacts
N-Acyl ko O-Acyl amino acid ko pepyl-coepaldzed acybation na amine ko hydroxyl ƙungiyoyi tare da kitse acid. Rahoton farko a kan mafi yawan launuka-catalyzed kira na amino acid din amino acide ya yi amfani da Candin Antarct 25% zuwa 90% ya danganta da Amino acid. An yi amfani da ethyl na Ketone a matsayin sauran ƙarfi a wasu halayen. Vonderenen et al. Hakanan aka bayyana lipase da kariya da catalyzed n-acyby halayen amino acid, furotin hydrolysates da / ko abubuwan gina jiki suna amfani da cakuda ruwa da / ruwa) da methyl butyl ketone.
A farkon zamanin, babban matsalar tare da enzyme-coatszed kira na Aas shine ƙarancin amfanin ƙasa. Bisa ga yara et al. Yawan amfanin gona na n-tetrarrusoyl amino acid shine kawai 2% -10% ko bayan amfani da launuka daban-daban da kuma shiryawa a 70 ° C na yawa. Montet et al. Hakanan kuma ci karo da matsaloli game da low yawan amfanin ƙasa na amino acid a cikin synth Lysine amfani da kits da mai kayan lambu. A cewar su, matsakaicin yawan amfanin ƙasa shine 19% a ƙarƙashin yanayin kyauta da kuma amfani da abubuwan haɗin gwiwar kwayoyin halitta. An ci karo da wannan matsalar ta hanyar haihuwa et al. A cikin syntharis na N-cbz-l Lysine ko N-CBZ-Lysine methyl Etster masu galihu.
A cikin wannan binciken, sun ce yawan yawan amfanin ƙasa na 3-o-Tetrownecanoyl-L-Serine ya kasance 80% lokacin amfani da merine da novozyst a cikin yanayin da za a iya kariya. Nagao da Kittin da Kito sun yi karatun o-wactation na L-Serine, L-Eyerinine, L-Teaniida da L-Tyrosine a lokacin da ake amfani da Lipaserine da L-Serine sun kasance kaɗan, yayin da babu wani achallation na L-BABUSI DA GOMA SHI.
Yawancin masu bincike sun goyi bayan amfani da amfani da tsada da sauri don substrates don syntharis na Aas. Soo et al. Da'awar cewa shirye-shiryen Surfactants mai-Pram mai suna aiki mafi kyau tare da rashin daidaituwa Lipoenzyme. Sun lura cewa yawan amfanin ƙasa zai zama mafi kyau duk da lokacin cinyewa lokaci (kwana 6). GerivA et al. Binciken tsarin da aka bincika da kuma aikin na yau da kullun na ASCL AS dangane da methonine, na ciyayi, phenylglycine da phenylglycine da phenylglycine da phenylglycine da phenylglycine a cikin cakuda cyclic / tsere. Pang da Chu sun bayyana synthari acid na amino aci aci acid da DicarBoxylic acid a cikin Maganin Amino acid Elyamide Cossers
Cantaeuzene da Guerreiro sun ba da rahoton cewa ƙungiyoyin cocboxylic acid na boc-al oh da boc-oh kuma bochlatethane da kibura, 4b (sephroose 4b) a matsayin mai kara kuzari. A cikin wannan binciken, da amsawar boc-shiru tare da kashin giya har zuwa carbons 16 sun ba da amfanin gona mai kyau (51%), yayin da carbons masu dacewa na 63% [64]. 99.9%) A da ake samu a cikin 58% zuwa 76%, wanda samuwar Amide Conts tare da kashin-sarkar giya da CBZ-Arg-ome, inda Papain ya yi aiki a matsayin mai kara kuzari.
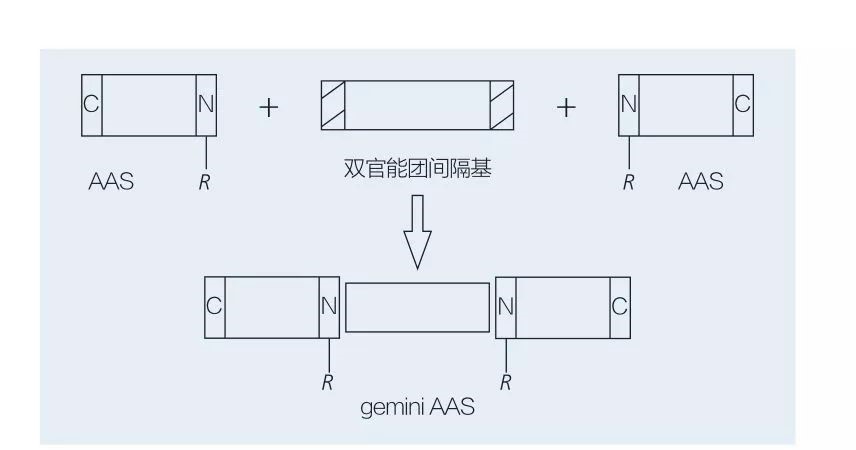
5.2.2 Sythesis na Gemini-tushen amino acid / peptide Surfacts
Amino acid Trimin Surfacts ya ƙunshi madaidaiciya-sarkar aas kwayoyin halitta da aka haɗa da juna-da-kai ga juna ta hanyar rukunin sararin samaniya. Akwai dabaru 2 na musamman don chemoenzymatic na chemoenzymatic na Gemini-Rubuta Surahacts na tushen amino acid (Figurs 6 da 7). A cikin Hoto na 6, 2 ana mayar da su a matsayin fili a matsayin ƙungiyar sararin samaniya sannan kuma an gabatar da ƙungiyoyin Hydrophobic. A cikin Hoto na 7, tsarin sarkar 2 madaidaiciya an haɗa shi kai tsaye tare da ƙungiyar mai ma'ana.
Farkon ci gaban enzyme na enzyme-catalalzed kira na Gemini Lipoamino acid ya shafi ta hanyar haihuwa et al. Yosimura et al. Binciken tsarin kira, adsorption da watsa na tushen jerin gwano na tushen Gemini acid dangane da Cystine da N-Alkyl bromie. An kwatanta Surfactutan da aka kwatanta da surfactics monomeric. Fausto et al. Aka bayyana syntharis na tushen anionic urea-tushen monomeric aas l-cystine, l-scheroine, l-cystine, l-sarstine, lethibrium surfaroaline da tsayayyen-jihar mai kyalli da na bayyana. An nuna cewa darajar CMC na Gemini ya ragu ta hanyar kwatanta Monomer da Gemini.
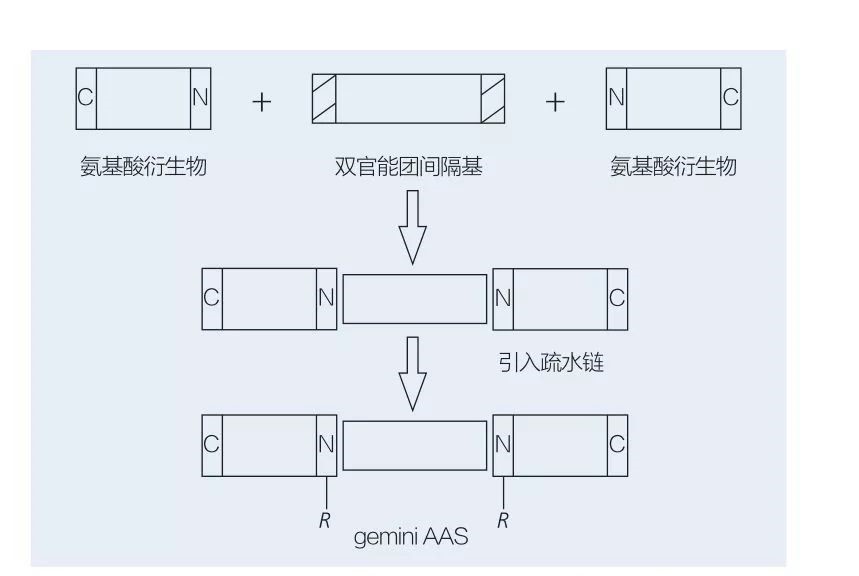
Fig.6 na Ginani Aas Amfani da AA NUFIN DA ASA, ta hanyar saka kungiyar ta Hydrophobic
Fig
5.2.2.3 kira na glycololorerifid amino acid / peptide surfaceants
Amincey amino acid / peptide surfactants sabon aji ne na Lipid amino acid dinsa ne, sarƙoƙi mai kitse tare da amino acid da bondror daya da hannu. Haɗin waɗannan Surfactant suna farawa da shirye-shiryen etarels na amino acid a ɗaukaka yanayin zafi da kuma a gaban mai mai mai kara acidic (misali BF 3). Enzyme-catalalzed kiras (ta amfani da hydroases, kariya da lipases a matsayin mai conlysts) shima mai kyau zaɓi (Hoto 8).
Enzyme-catalalzed kira na DILLahimRin arginaly glycastides ta amfani da Papain da aka ruwaito. Hythesis na diaryllglycerol ester conjugates daga ACEYLAGINE DA GASKIYA DA IYALIYAR Kayayyakinsu na likitancinsu.
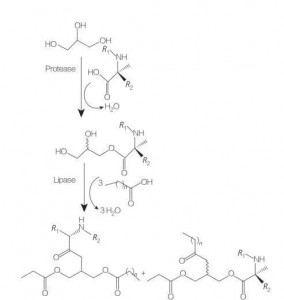
Fig.8 kira na Mono da Diarylglyce Amino acid conjugates
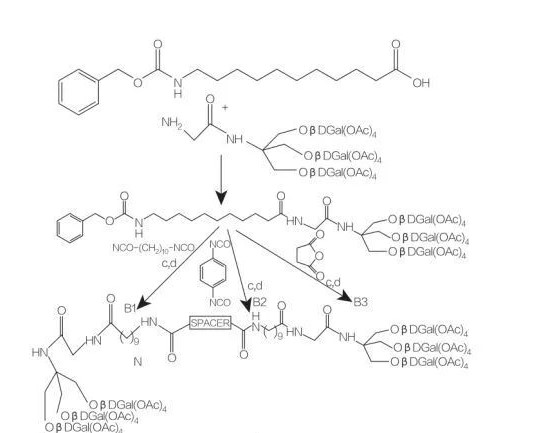
Spacerer: NH- (ch2)10-Nh:
Spacerer: NH-C6H4-Nh:
Spaceer: ch2-S2: Stroupoundb3
Fig
5.24.4 Synthesis na amino acid / peptide surfacts
Amino acid-tushen ampla-nau'in amphiphile sun ƙunshi amino acid guda biyu waɗanda ke da alaƙa da sarkar ruwa. Franceschi et al. Aka bayyana syntharis na nau'in Bola-nau'in ampelhiles tare da Amino acid (D- ko L-Alnine ko L-Alkyl Sarkar Damanni daban-daban da kuma bincika ayyukansu daban-daban. Suna tattauna da kira da kuma tsoffin labaran Bola-nau'in ampelhiles tare da kabad din amino acid (amfani da ko dai wani sabon salo ko kuma cunkoso. Ba a saba da β-amino acid din da aka yi amfani da shi na iya zama amoooacid, Azidothymin (azidbneymind amino acid, da kuma amino giya ya samu daga azti (Hoto na 9). Hyuntheis na Sythmetrical BOLAC-nau'in samar da ishiphiles daga Tris (Hydroxymethyl) Aminomethane (tris) (Hoto na 9).
06 Physicochemical Phys
Sanannen sanannen Surfactantsan asalin amino aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci aci acid (AAS) ya bambanta da kuma ingantaccen aiki a cikin ruwa da yawa (ingantaccen aiki).
Dangane da kayan Surfactant na amino acid (misali tashin hankali na yau da kullun, CMP, halayen AAS bayan da takwarorinsu na al'ada ya fi gaban takaddama na al'ada.
6.1 masu cikakken hankali na micelle (CMC)
Millal mai mahimmanci yana ɗayan mahimman sigogi na Surfacts da Governs da yawa daga cikin kayan aikin hydrocachon, da sauransu gaba ɗaya, da sauransu gaba ɗaya, da yawa. Surfacts dangane da amino acid yawanci suna da darussan CMC idan aka kwatanta da surfactants na al'ada.
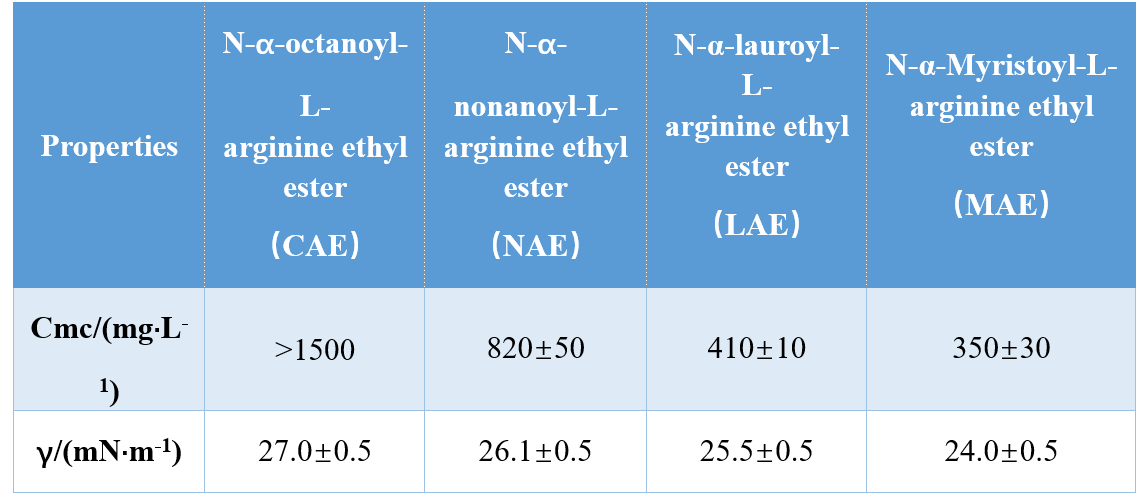
Through different combinations of head groups and hydrophobic tails (mono-cationic amide, bi-cationic amide, bi-cationic amide-based ester), Infante et al. Haɗin kai na AAs guda uku na AAS kuma nazarin CMC da γcmc (tashin hankali na CMC), yana nuna cewa ka'idojin CMC da γCMC dabi'u sun ragu da tsayin wutsiya na ruwa. A wani binciken, ringa da mhatre da mhat farta cewa cmc of N-α αacginine surfactants ya ragu tare da ƙara yawan yawan wutsiya carbon atomrophobobic carbon atomrophobo na ruwa (Table 1).
Yosimura et al. investigated the cmc of cysteine-derived amino acid-based gemini surfactants and showed that the cmc decreased when the carbon chain length in the hydrophobic chain was increased from 10 to 12. Further increasing the carbon chain length to 14 resulted in an increase in cmc, which confirmed that long-chain gemini surfactants have a lower tendency to aggregate.
Fausto et al. An ruwaito samuwar gauraye micres a cikin mafita mafi kyau na Surmin Surfactants na anionic dangane da cystine. An kuma gwada Surminnants din Gemini tare da Surfactants mai dacewa na al'ada (c 8 cs). An ba da rahoton dabi'u na CMC na cakuda gaurayowar da aka ruwaito su ƙasa fiye da waɗanda ke tsarkakakkiyar surfaceants. Gemini Surfactants da 1,2-Glycetanoyl-3-glyceryl-3-phosphhocholine, ruwa-glisphocholine, yana da phospholipid, yana da CMC a matakin milimollar.
Shrestsha da Aramaki sun bincika samuwar tsutsotsi na Viscoalastic-kamar miceles a cikin mafita na hadewar hade da hadayowar hade da kayan kwalliya. A cikin wannan binciken, an gano n-dodecyl glutamate don samun babban zafin jiki na Krefft; Koyaya, a lokacin da aka dakatar da amino acid L-Lysine, ya haifar da miceles da kuma maganin ya fara zama kamar ruwa na Newtian a 25 ° C.
6.2 Kyakkyawan Siyarwa mai Kyau
Kyakkyawan Solyarfin ruwa na Aas ya kasance saboda kasancewar ƙarin haɗin kai-nH. Wannan yana sa Aas ƙarin bishara da kuma tsabtace muhalli na yanayin al'ada. Solyarfin ruwa na n-acyl-l-glutamic acid ya fi kyau saboda ƙungiyoyin carboyl 2. Karfin ruwa na CN (Ca) 2 yana da kyau saboda ƙungiyoyi 2 na ionic, wanda ke haifar da damar kwayoyin cuta a cikin ƙananan taro.
6.3 krafft zazzabi da krafft Point
Za'a iya fahimtar krafft a matsayin takamaiman halayyar silurration wanda ƙila ya ƙara sama da yawan zafin jiki. Ionic Surfactantantant suna da hali don samar da ingantaccen hydrates, wanda zai iya haifar da ruwa. A wani zazzabi na musamman (abin da ake kira Krafft zazzabi), mai ban mamaki da kuma dakatar da ƙara haɓakar abubuwan da ake ciki ana lura dashi. Matsayin krafft na ionic surfactant shine Krafft zazzabi a CMC.
Wannan yawanci ana ganin halayen da aka saba gani don Surfactants na ionic kuma ana iya bayanin su kamar haka: Solubity na Surfactant Free monactally yana da iyaka saboda sannu-sannu a hankali samuwar. Don tabbatar da cikakken solive, ya zama dole don shirya tsari surfactant a yanayin zafi sama da krafft.
Za'a yi nazarin Krafft na Aas kuma idan aka kwatanta da na Tudet na al'ada na AAS kuma ya yi nazarin yanayin tarihin (OHTA × iri-iri (OHTA EXDIC N-Hexadecanoyl Aas kuma tattauna dangantakar tsakanin yanayin krafft da sauran ragowar amino acid.
A cikin gwaje-gwajen, an gano cewa Krafft zazzabi na N-hexadecanoyl Aas ya karu da raguwar sharar da (tare da zafin rana), amma da zafin rana da phenylaline). An gama da cewa a cikin dukkan alanine da tsarin phenylalanine, hulda dl ya fi ƙarfin ma'amala a cikin m siffofin n-hexadecanoyl AAS kullum.
Brito et al. An ƙaddara zafin jiki na Krafft uku na jerin abubuwan da aka dangera Up acid din amintaccen Iion da kuma gano wanda ke canzawa, daga 67 ° C), daga 47 ° C. Kasancewar shaidu biyu da marasa ilimi suna cikin jerin abubuwan da aka ba da dogon lokaci da aka haifar da raguwa mai mahimmanci a cikin zazzabi Krafd. N-Dodecyl Glutamate an ruwaito yana da yawan zafin jiki na Kreff. Koyaya, neuts tare da Amino acid L-Lysine ta haifar da samuwar miceles a cikin mafita da ke nuna cewa yayin da Newtian ruwa a 25 ° C.
6.4
A cikin tashin hankali na surfactants yana da alaƙa da jerin sarkar na hydrophobic. Zhang et al. Kulla da tashin hankali na sodium cocoyl glycate (25 ± 0.2) ° C da kuma yanke shawarar farfaɗo a cikin CMc 33 Mn1, CMC kamar 0.21 MMOL-L -1. Yosimura et al. An ƙaddara tashin hankali na 2C n cays da aka gyara aminci aci aci aci aci aci aci aci aci acid masarauta na 2c n cys-tushen wakilai masu aiki. An gano cewa tashin tashin hankali a CMC Rage tare da ƙara tsawon sarkar (har sai n = 8), yayin da aka sake juyawa ga surfactants tare da n = 12 ko tsayi sarkar tsawo.
Tasirin Cac1 2 A kan tashin hankali na Dicarbox. A cikin waɗannan karatun, an ƙara Cac1 2 zuwa mafita na ruwa na Dicarbox (C12 Malna 2, C12 Aspna 2, da C12 Gluna 2). An kwatanta darajunan Filato bayan da aka kwatanta CMC kuma an gano cewa tashin hankali na sama ya ragu a sosai low cac1 2 maida hankali. Wannan ya faru ne saboda tasirin ions a kan tsarin Surfactant a cikin binciken gidan mai. A farfajiya na silts na salts na n-dodecylaminarate da n-durdocylamaspartrate, a gefe guda, har ma kusan akai har zuwa 10 MMOL-l -1 Cac1 2 maida hankali. Sama sama da 10 MMOL-L -1, tashin hankali yana ƙaruwa sosai, saboda samuwar hazo na alli na Surfactant. Don sondium gishiri na n-dodecyl glutamate, matsakaici na Cac1 2 ya haifar da raguwa a cikin tashin hankali na CAC1 2 Ba a tabbatar da canje-canje masu mahimmanci.
Don ƙayyade maɓallin adsetics na Gemini-Type Aas a cikin binciken mai-ruwa, tashin hankali mai tsauri, an ƙaddara ta amfani da matsakaicin matsin lamba mai kwari. Sakamakon binciken ya nuna cewa don mafi dadewa lokacin gwaji, 2C 12 mai rikitaccen tashin hankali ba ya canzawa. Rage na tashin hankali mai tsauri ya dogara ne kawai akan taro, tsawon wutsiyoyi na hydrophobic, da kuma yawan wutsiyoyi na hydrophobic. Theara taro na surfactant, rage tsawon sarkar tsallake kamar yadda yawan silins ya haifar da samun saurin lalata. Sakamakon da aka samu don mafi girma taro na C n cys (n = 8 zuwa 12) an gano cewa yana da kusanci da hanyar Wilhelmy da Wilhelmy.
A wani nazarin, tashin hankali mai tsauri na Sodium Dilural Cystine (SDLC) da sodium dridecamino cystine an ƙaddara shi ta hanyar mafi girman hanyoyin ruwa na ruwa. An kara daukar martani na disulfode ta wasu hanyoyin kuma. Additionarin ƙari na Mercaptoethanol zuwa 0.1 Mmol-L -1sdlc bayani ya haifar da saurin karuwa a cikin tashin hankali na 34 mn-m -1 zuwa 53 mn-m -1. Tun da Naclo zai iya oxidicize bangarorin SDLC zuwa kungiyoyin acid na sulfonic, babu tara a cikin maganin 0.1 MM1 na--1. Sakamakon watsa Haske na Iskiro da ƙarfin watsa sakamako wanda aka nuna cewa babu tara tara a cikin mafita. An gano fargaba na SDLC na karuwa daga 34 mn-m -1 zuwa 60 mn-m -1 a tsawon 20 min.
6.5 Binary Computances
A cikin kimiyyar rayuwa, kungiyoyi da yawa sun yi nazarin kadarorin hadin gwiwar cawanic aas (Surfinan tushen tushen da ke haifar da cewa wannan kyakkyawan kayan da ke haifar da wucewar hulɗar wutar lantarki.
6.6 TARIHIN TAFIYA
Wynlic Haske mai watsa ruwa ana amfani dashi don ƙayyade kaddarorin tarin amino acid da kuma Surfactants a bayyane na m diamita dh (= 2r h). Taro da aka kirkira ta C n Coys da 2cn cys suna da girma kuma suna da babban rarraba sikelin idan aka kwatanta da wasu surfactants. Duk Surfactants ban da 2C 12 cys yawanci suna haifar da tara kusan 10 nm. Girman micelle Surfactants ne mai mahimmanci fiye da waɗanda na takwarorinsu na monomeric. Karuwa a cikin sarkar sarkar hydrocarbon ma yana haifar da karuwa a girman micelle. OHTA et al. Aka bayyana kaddarorin hadari na mutum daban daban na n-dodecyl-Alanine Tetrametlmonium a cikin bayani mai yawa. Iwahashi et al. An bincika ta Dichroism na Dichroism, NMR da tursasawa osmometry osmometlic-l-drinineoy acid na n-drinine da tetrahanoyl-l-drininetrile, acetonrile, 1,4-dioxane da 1,2-Dichloroethane) tare da Dichroism Proardsisism mai bincike, NMR da tursasawa ostmometry.
6.7 Adsorption Adsorption
Adsorction Interfacial na Sepo acid-tushen keɓaɓɓen abubuwan da aka danganta da kuma kwatankwacin da takwaransa na al'ada shine ɗayan kwatancen bincike. Misali, kadarorin adsorction na esfers na asso acid dino acid dino acid din da aka samu daga barin kuma an bincika shi kuma an bincika. Sakamakon ya nuna cewa bari kuma LEP ya nuna ƙananan wuraren da ke cikin gida a cikin binciken mai-taya da kuma a cikin ruwa / hexane dubawa, bi da bi.
Bordes etles et al. Binciken halayyar da aka warware da adsorption a cikin binciken mai-gas na Dicarboxboxy na Dicarbox, da kuma zinomalon a matsayin carbon a tsakanin ƙungiyoyin carbon guda biyu, bi da bi). Dangane da wannan rahoton, CMC na Surfactantsan wasan kwaikwayo na Dicarboxylants ya kasance sau 4-5 sama da na monocarboxylated gishiri na glycine gishiri. Wannan an danganta wannan ne ga samuwar hydrogen na hydrogen tsakanin tsinkayen Dicarboxylants da makwabtan kwayoyin a cikin tsarin koyarwa a ciki.
Halin Mataki na 6.8
An lura da matakan kwayoyin halitta na yau da kullun ana lura da su don sinadantattun abubuwa a sosai babban taro. Kwayoyin halittu masu santsi tare da manyan rukunin kai tsaye suna iya samar da tarin karar da karami mai kyau. Marques et al. Nazarin yanayin lokaci na 120s12 / 12serser da 8lysser (duba Hoto na 10lys12 / 16/16 tsarin tsarin rabuwa da covers10 / 16/2ser tsarin yana nuna wani lokaci na 8lys10 / 16/2ser tsarin yana nuna wani lokaci na 8lys10 / 16/16/16 Ya kamata a lura cewa ga yankin vestic na ves1012 / Vesicles na yau da kullun tare da miceles, yayin da tsarin ves8 / 16 sashi yana da vesicles kawai.
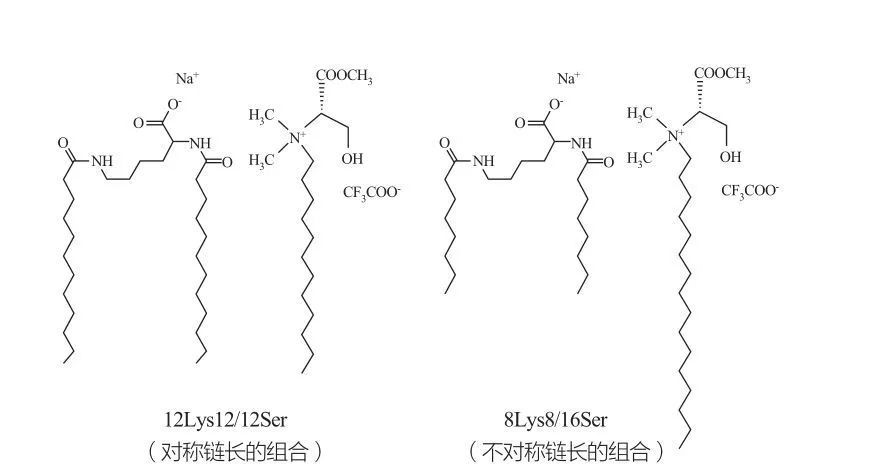
Zaman catanionic na Lysine- da Surfactants na Surka: Symmetric 122Ts12 / isersser Haɗa (hagu) da asymmetrics (dama)
6.5 Kwarewar emulsifonywa
Koouchi et al. Ya bincika iyawar emulsifin, tashin hankali na addinai, ban sha'awa, da danko. A kwatankwacin Sirteacts na yau da kullun (na al'ada noonsic da kuma amphoteric na al'ada na al'ada), sakamakon ya nuna cewa Aas na da ikon emulsiphy fiye da surfactants na al'ada.
Baczko et al. Rashin daidaito anionic amino acid kuma an bincika abubuwan da suka dace a matsayin kayan kwalliya na Chural mai zagaye na NMR. Jerin jerin Ingamarate-Suldanate-L-Phe ko L-ALAVIVES tare da wutsiyoyi daban-daban (Pentyl ~ ~ titradanco acid tare da o-sulfobenzophoic anhydride. Wu et al. Sondurized sodium salts na n-Fatty acyl Aas daBinciken ikonsu na Emulsification a cikin emulsions mai cikin mai, kuma sakamakon ya nuna cewa wadannan surfactants sun yi kyau sosai tare da ethyl acetate a matsayin mai da n-hexane a matsayin mai.
6.10 ci gaba a cikin kira da samarwa
Za'a iya fahimtar juriya da ruwa a matsayin ikon Suttacts don tsayayya da kasancewar ions kamar alli da magnesisium a cikin ruwa mai wuya, watau, ikon guje wa hazo zuwa alli a cikin alli. Surfactants tare da babban ruwa mai wuya ruwa suna da amfani sosai ga kayan wanka da samfuran kulawa na mutum. Za'a iya kimanta juriya na ruwa mai wuya ta hanyar lissafin canji a cikin warwarewar da kuma ayyukan surfacal a gaban alƙawarin alli.
Wata hanyar don kimanta ƙarfin tsayayyen ruwa shine a lissafta kashi ɗaya ko grams na Surfactant don ɓoyayyen sabulu don a watsa shi cikin ruwa. A cikin yankuna tare da ruwa mai wuya, babban maida hankali na alli da magnesium ions da abun ma'adinai na iya yin wasu aikace-aikace masu wahala. Sau da yawa ana amfani da indium ion kamar yadda keɓaɓɓe na surfactant Surfactant Surfactant Suretant. Tun lokacin da divental ion ya daure wa duka sirrin kwayoyin halitta, yana haifar da surfactant don yin hazo mafi sauƙin warware matsalar da ba zai yiwu ba.
Nazarin jurewar Aas da aka nuna cewa acid da kuma hatsarancin ruwa na Carboxyl, da kuma acid da kuma tsayayyen ruwa da ruwa da wuya a kan karagar da ke tsakanin ƙungiyoyin carbox guda biyu. Umurfin acid da juriya na ruwa shine C 12 glycast <c 12 a aspartate <c 12 glutamate. Kwatanta da Dicarboxylated Dicar Bond Ude Bond da Dicarboxyal amino surfactant, bi da bi, an gano cewa Loster kewayon ya zama da ƙari na adadin adadin acid na acid da ya dace. Dicarboxylance N-Alkyl amino acid n-alkyl amino acid n-alkyl amino acid yana nuna tasirin chelating sakamako a gaban alƙawalin ions, da C 12 mai aspartate kafa farin Gel. c C (Glutemate ya nuna babban aiki na sama a babban ca 2+ maida hankali kuma ana tsammanin za a yi amfani da shi a cikin layin teku.
6.11 Dankwasawa
Rashin damuwa yana nufin iyawar Surfactant don hana coalescence da gyada mai narkewa a cikin bayani a cikin bayani.Rashin daidaituwa muhimmin abu ne na Surfactants wanda ya sa suka dace da amfani a cikin kayan wanka, kayan kwalliya da magunguna.Wani wakili na watsawa dole ne ya ƙunshi ester, ether, don haɗin kai tsakanin ƙungiyar hydrophobic da tashoshin rudani (ko a tsakanin madaidaiciyar sarkar hydrophobic).
Gabaɗaya, surfactants din anionic kamar suilamates sulfates da kuma Surfacting Surfacts da Surfactants na Amidosulffoobeine suna da tasiri musamman kamar watsawa don sabulu soaps.
Yawancin kokarin bincike da yawa sun yanke shawarar wakoki na Aas, inda aka samu n-Lauroyl Lysine talauci da ruwa da kuma wahalar amfani da tsarin kwaskwarima.A cikin wannan jerin, N-Acyl-musayar asali amino acid suna da abubuwan ban sha'awa kuma ana amfani da su a cikin masana'antar kwaskwarima don inganta tsari.
07 mai wahala
Tsarin Sadarwar al'ada, musamman Cashic Surcantants, suna da guba ga kwayoyin ruwa. Abubuwan da suka faru da guba shine saboda sabon abu na adsorption-ion hulɗa na Surfactants a wayar salula. Rage CMC na Surfactants yawanci yana haifar da saurin adfact of Surfactants, wanda yawanci yana haifar da cutar masu guba sosai. Karuwa a tsawon hydrophobic sarkar Surfactant shima yana haifar da karuwa a cikin Surfactant m cutexicity m.Yawancin Aas suna da ƙarancin gaske ko marasa guba ga mutane da muhalli (musamman ga kwayoyin marine) kuma sun dace da amfani azaman kayan abinci, magunguna da kayan kwalliya.Yawancin masu bincike sun nuna cewa Suriyar Amino acid mai laushi ne mai laushi da rashin jijiya. An san Surfactants na Arginine da ba su da ƙarancin isarwa fiye da takwarorinsu na al'ada.
Brito et al. Nazarin Phyicochemical da guba na tushen masana'antar Amino acid da [hyr), wingroxyproline (hyr), mitar vesine (lms), mitar vesine Suna haifar da rigunan murƙushewar bromide (DTab) / Lys-abubuwan hawa da / ko Sers-abubuwan maye, suna nuna cewa duk AAS da Surfactawy na al'ada DTab.
Rosa et al. Binciken da ya shafi ɗaukacin (Ass) na DNA zuwa DNA-TOMI acid Vesics. Ba kamar Surfin Cationic na al'ada ba, wanda sau da yawa ya bayyana cewa mai guba, hulɗa na Cimic Amino acid Surfacid yana bayyana ba shi da guba. ASSIC AAS ya dogara ne da arginine, wanda ba a tsayayye vesicles a hade tare da wasu surfactant din anionic. Hakanan ana amfani da cututtukan cututtukan cututtukan amino acid na masu hana su sunfigior da ba su da guba. Wadannan surfactants suna cikin sauƙin hadewa tare da tsarkakakke (har zuwa 99%), low cost, cikin sauki da sauƙaƙe, kuma gaba ɗaya a cikin kafofin watsa labarai. Karatun da yawa sun nuna cewa Suro acid Suro acid Surahacts suna da fifiko a cikin hanzari.
A cikin binciken da ya gabata, Pininchi et al. bayar da rahoton bayanin martaba mai gamsarwa na rhamolipids idan aka kwatanta da surfactants na al'ada. Ruhnolipids an san su don yin amfani da haɓaka. Sun kuma ba da rahoton sakamakon sakamakon Ruhampids a kan batun macromelecular kwayoyi.
08 Antimicrobial Aikin
Ana iya kimanta ayyukan antimicrobial na Surfactants ta ƙarancin infihitory maida hankali. An karanta aikin antimicrobi da aka danganta da Surfactantsan Suriactants na Arginine daki-daki. An gano ƙwayoyin cuta mara kyau don zama mai tsayayya da Surfactants-tushen Surfactants da ƙwayoyin cuta mai kyau. Aikin antimicrobial na Surfactants yawanci yakan ƙaru da gaban hydroxyl, cyclopropropaney a cikin sarƙoƙin ACYL. Castillo et al. ya nuna cewa tsawon ɗaukar kaya da kuma tabbataccen cajin HLB (ma'aunin rudani) na kwayar halittar, kuma waɗannan suna da tasiri a kan iyawar su na rushe membranes. Nα acylarine methyl ester wani muhimmin aji ne na Cayin na Cayin tare da aiki mai santsi tare da amfani da shi kuma yana da ƙarancin ƙarfi ko kuma mai guba ne. Nazarin a kan hulɗa na Nα-acylginine methyl Estterants tare da 1,2-ditetlristlcholine, kuma tare da halittu masu rai a gaban ko rashin shinge na waje Nuna cewa wannan aji na surfactants yana da maganin rigakafi mai kyau sakamakon binciken ya nuna cewa Surfactants suna da aikin ƙwayoyin cuta na ƙwayoyin cuta.
09 rheological kaddarorin
Abubuwan da kaddarorin Surfactant suna taka muhimmiyar rawa wajen tantance kuma suna tsinkayar aikace-aikacensu a cikin masana'antu daban-daban, hakar mai, kulawa da kayayyakin mai da kayayyakin mai. An gudanar da karatun da yawa don tattaunawa game da dangantakar tsakanin Viscoecaity na Surci acid Surfactants da CMC.
10 Aikace-aikace a cikin masana'antar kwaskwarima
Ana amfani da Aas a cikin nau'ikan samfuran kulawa na sirri da yawa.Potassium n-cocoyl glycate an samo shi da ladabi a kan fata kuma ana amfani dashi a cikin tsabtace fuska don cire sludge da kayan shafa. N-Acyl-L-Glutamic acid rukuni biyu ne, wanda yasa shi karin ruwa mai narkewa. Daga cikin waɗannan Aas, Aas dangane da C 12 acid acid ana amfani dashi sosai a cikin tsabtace fuska don cire sludge da kayan shafa. Aas tare da sarkar AS 18 kamar yadda emulsifiers a cikin samfuran kula da fata, kuma ana iya amfani da salts na fata waɗanda ba za a iya amfani da su ba kuma saboda haka za a iya amfani da su a cikin samar da kayayyakin kulawa na baby. N-Lauryl-tushen abin haƙoran haƙora suna da kyakkyawar hanyar wucin gadi mai kama da sabulu da ƙarfi-infichibing.
A cikin 'yan shekarun da suka gabata, zaɓi na surfactants ga kayan kwaskwarima, samfuran kulawar mutum da magunguna sun mayar da hankali ga ƙarancin guba, m, ladabi ga taɓawa da aminci. Masu amfani da waɗannan samfuran suna sane da yiwuwar haushi, toxicty da abubuwan muhalli.
A yau, ana amfani da AAS don tsara shafukan da yawa, gashin gashi da kuma soaps wanka saboda yawancin takwarorinsu na gargajiya a cikin kayan kwalliya da kayayyakin kulawa na mutum.Surfacts na tushen kariya suna da kyawawan kaddarorin da ake buƙata don samfuran kulawa na mutum. Wasu Aas suna da damar samar da fim, yayin da wasu suke da kyawawan abubuwa masu kyau.
Amino acid suna da mahimmanci a zahiri wanda ke faruwa a zahiri a cikin Statum Corneum. A lokacin da sel Epidermal mutu, sun zama wani ɓangare na Stramum Corneum kuma sunadarai sunadarai a hankali lalata amino acid. To, waɗannan amino acid suna ci gaba cikin Stram Corneum, inda suka sha mai ko abubuwa masu kitse, don haka ya inganta elarmality na fata. Kimanin kashi 50% na na moisturizing factor a cikin fata ya ƙunshi amino acid da kuma purrolidoneone.
Collagen, sashi na al'ada na gama gari, ya kuma ƙunshi amino acid ɗin da ke kiyaye fata taushi.Matsalolin fata kamar m da lalata sun kasance a cikin babban sashi zuwa rashin amino acid. Nazarin daya na binciken da aka nuna cewa hade amino acid tare da maganin shafawa mai sauƙin ƙonewa yana ƙonewa, kuma wuraren da abin ya shafa sun dawo da yanayinsu na al'ada ba tare da zama kawun Keliki ba.
Hakanan an gano Amino acid sosai wajen kula da yankan da suka lalace.Dry, gashi mara kyau na iya nuna raguwa a cikin maida hankali ga amino acid a cikin babban rauni mai lalacewa. Amino acid suna da ikon shiga cikin m a cikin shannand gashi kuma sha danshi daga fata.Wannan ikon amintaccen tushen Sadar da Amino aci aci aci aci acid yana sa su zama mai amfani a cikin shamfu, gashin gashi, gashin gashi, da gaban amino acid yana sa gashi ya zama mai ƙarfi.
11 Aikace-aikace a cikin kayan kwalliya na yau da kullun
A halin yanzu, akwai buƙatar ci gaba don ingantaccen kayan daskararren amino acid a duk duniya.Aas sanannu ne don samun ingantacciyar tsaftacewa, iyawa da take shimfiɗawa da masana'anta masu laushi, wanda ya sa suka dace da kayan abinci na gida, shamfu, wanke jiki da sauran aikace-aikacen.An ruwaitaccen ASPARTIC ADMHoteric Aas an ruwaito ya zama babban abin sha mai kyau tare da Cheelating Procroes. Ana amfani da amfani da kayan abinci na wanka wanda aka samo na n-alkyl-β-aminethoxy an samo shi don rage zafin fata. An ruwaito tsarin wanka na ruwa wanda ya ƙunshi n-cocoyl-β-aminopripionate ya zama mai tasiri na rigakafin don sinadarin mai a kan ƙarfe saman. Aminocarboxylic acid Surfactant, c 14 Chohch 2 Nhch 2 Nhch 2 Coona, an kuma yi amfani da shi don tsaftace abubuwa, n-aminoproxy mai iyawa da haka ne n-aceproxy iyawa.
Shirye-shiryen kayan wanka dangane da N- (N'-Long-Alanin Centl-β-Alanine da kwanciyar hankali, sassauƙa kera alasinine mai kyau. Kao ya bunkasa wani abu na wanka dangane da n-acyl-1-Alnine da kuma bayar da rahoton low mackation, babban ruwa da kuma karfin cirewa da kuma ƙarfin ruwan sha da kuma ƙarfin cirewa da kuma ƙarfin ruwan sha da kuma ƙarfin cirewa da kuma girman ƙarfin iska da kuma ƙarfin cirewa da kuma ƙarfin cirewa da kuma ƙarfin tsayayyen ƙazanta da kuma ƙarfin ƙwayar ruwa da kuma ƙarfin ƙwayar ruwa da kuma ƙarfin ƙwayar ƙwayar ruwa.
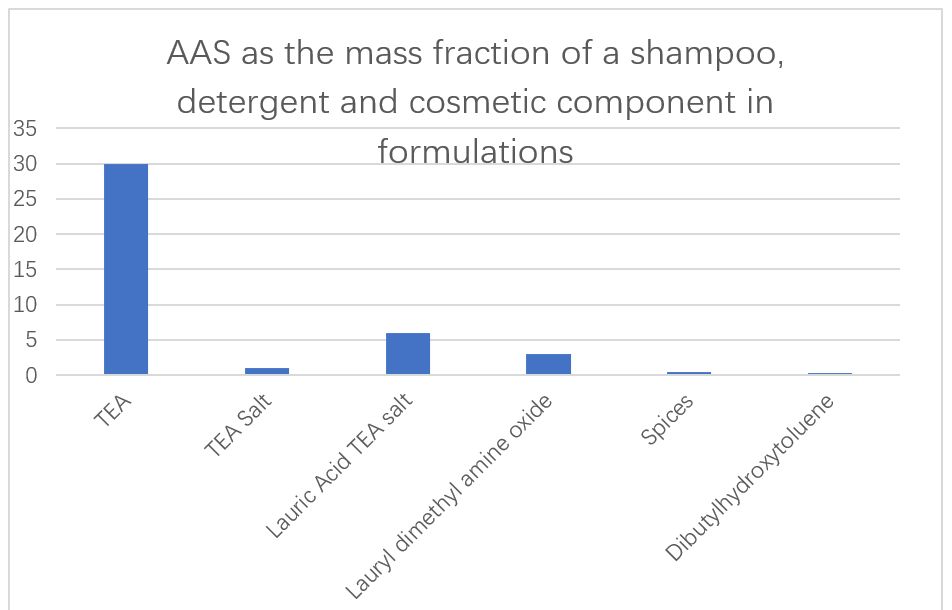
Kamfanin Japanomoto Ajinomoto yana amfani da ƙarancin guba mai sauƙi da sauƙi mai amfani da kayan masarufi, L-arginine da L-Lyne da L-Lysine da L-Lysine da L-Lysine da L-Lysine da L-Lyne da L-Lysine da L-Lysine da L-Lysine da L-Lyne da L-Lysine da L-Lysine da L-Lyne da L-Lysine da L-Lyne da L-Lyne da L-Lysine da L-Lysine da L-Lysine da L-Lyne da L-Lysine da L-Lyne da L-Lysine da L-Lysine da L-Lysine da L-Lyne da L-Lysine da L-Lyne da L-Lysine da L-Lyne da Lysine a matsayin babban sinadari a cikin shamfu, kayan wanka da kayan kwalliya (Hoto na 13). Ikon ƙara enzyme a cikin kayan wanka don cire ƙuruciyar furotin. N-Acyl Aas da aka samo daga Glutamic acid, Alana, methylglycine, Metine da Ajiyayyen acid don amfanin su a matsayin kyakkyawan ruwa a kan mafita mafita. Wadannan surfactants ba sa ƙara danko ko koda a yanayin zafi sosai, kuma ana iya canza shi cikin jirgin ruwan din da ya yi don samun kumfa mai ɗora.
Lokaci: Jun-09-2022

